Osmolality of Commercially Available Oral Rehydration Solutions: Impact of Brand, Storage Time, and Temperature
- PMID: 31261904
- PMCID: PMC6682936
- DOI: 10.3390/nu11071485
Osmolality of Commercially Available Oral Rehydration Solutions: Impact of Brand, Storage Time, and Temperature
Abstract
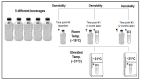
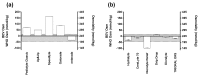
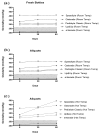
Oral rehydration solutions (ORS) are specifically formulated with an osmolality to optimize fluid absorption. However, it is unclear how many ORS products comply with current World Health Organization (WHO) osmolality guidelines and the osmotic shelf-life stability is not known. Therefore, the purpose of this investigation was to examine the within and between ORS product osmolality variation in both pre-mixed and reconstituted powders. Additionally, the osmotic stability was examined over time. The osmolality of five different pre-mixed solutions and six powdered ORS products were measured. Pre-mixed solutions were stored at room temperatures and elevated temperatures (31 °C) for two months to examine osmotic shelf stability. Results demonstrated that only one pre-mixed ORS product was in compliance with the current guidelines both before and after the prolonged storage. Five of the six powdered ORS products were in compliance with minimal inter-packet variation observed within the given formulations. This investigation demonstrates that many commercially available pre-mixed ORS products do not currently adhere to the WHO recommended osmolality guidelines. Additionally, due to the presence of particular sugars and possibly other ingredients, the shelf-life stability of osmolality for certain ORS products may be questioned. These findings should be carefully considered in the design of future ORS products.
Keywords: dehydration; electrolytes; euhydration; oral rehydration therapy; rehydration.
Conflict of interest statement
S.N.C. and R.W.K. are paid consultants to EHS and contributed to the research study concept and design, writing, and editing of the manuscript.
Figures
References
-
- King C.K., Glass R., Bresee J.S., Duggan C. Managing acute gastroenteritis among children: Oral rehydration, maintenance, and nutritional therapy. MMWR. Recomm. Rep. 2003;52:1–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

