Electrospun Polyvinylidene Fluoride-Based Fibrous Scaffolds with Piezoelectric Characteristics for Bone and Neural Tissue Engineering
- PMID: 31261995
- PMCID: PMC6669491
- DOI: 10.3390/nano9070952
Electrospun Polyvinylidene Fluoride-Based Fibrous Scaffolds with Piezoelectric Characteristics for Bone and Neural Tissue Engineering
Abstract
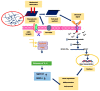
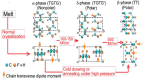
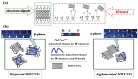
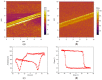
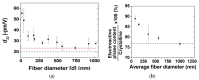
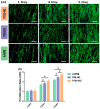
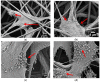
Polyvinylidene fluoride (PVDF) and polyvinylidene fluoride-trifluoroethylene (P(VDF-TrFE) with excellent piezoelectricity and good biocompatibility are attractive materials for making functional scaffolds for bone and neural tissue engineering applications. Electrospun PVDF and P(VDF-TrFE) scaffolds can produce electrical charges during mechanical deformation, which can provide necessary stimulation for repairing bone defects and damaged nerve cells. As such, these fibrous mats promote the adhesion, proliferation and differentiation of bone and neural cells on their surfaces. Furthermore, aligned PVDF and P(VDF-TrFE) fibrous mats can enhance neurite growth along the fiber orientation direction. These beneficial effects derive from the formation of electroactive, polar β-phase having piezoelectric properties. Polar β-phase can be induced in the PVDF fibers as a result of the polymer jet stretching and electrical poling during electrospinning. Moreover, the incorporation of TrFE monomer into PVDF can stabilize the β-phase without mechanical stretching or electrical poling. The main drawbacks of electrospinning process for making piezoelectric PVDF-based scaffolds are their small pore sizes and the use of highly toxic organic solvents. The small pore sizes prevent the infiltration of bone and neuronal cells into the scaffolds, leading to the formation of a single cell layer on the scaffold surfaces. Accordingly, modified electrospinning methods such as melt-electrospinning and near-field electrospinning have been explored by the researchers to tackle this issue. This article reviews recent development strategies, achievements and major challenges of electrospun PVDF and P(VDF-TrFE) scaffolds for tissue engineering applications.
Keywords: aligned fiber; electrospinning; neuron; osteoblast; piezoelectricity; polyvinylidene fluoride; polyvinylidene fluoride-trifluoroethylene; scaffold; stem cell; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




























References
-
- Acosta M., Novak N., Patel S., Vaish R., Koruza J., Rossetti R.A., Rodel J. BaTiO3-based piezoelectrics: Fundamentals, current status, and perspectives. Appl. Phys. Rev. 2017;4:041305. doi: 10.1063/1.4990046. - DOI
-
- Martins P., Lopes A.C., Lanceros-Méndez S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014;39:683–706. doi: 10.1016/j.progpolymsci.2013.07.006. - DOI
-
- Soulestin T., Ladmiral V., Dos Santos F.D., Ameduri B. Vinylidene fluoride- and trifluoroethylene-containing fluorinated electroactive copolymers. How does chemistry impact properties? Prog. Polym. Sci. 2017;72:16–60. doi: 10.1016/j.progpolymsci.2017.04.004. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

