In Vitro and In Vivo Antimalarial Activity of LZ1, a Peptide Derived from Snake Cathelicidin
- PMID: 31262018
- PMCID: PMC6669622
- DOI: 10.3390/toxins11070379
In Vitro and In Vivo Antimalarial Activity of LZ1, a Peptide Derived from Snake Cathelicidin
Abstract
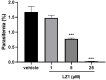
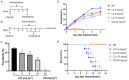
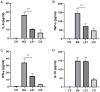
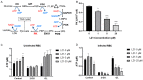
Antimalarial drug resistance is an enormous global threat. Recently, antimicrobial peptides (AMPs) are emerging as a new source of antimalarials. In this study, an AMP LZ1 derived from snake cathelicidin was identified with antimalarial activity. In the in vitro antiplasmodial assay, LZ1 showed strong suppression of blood stage Plasmodium falciparum (P. falciparum) with an IC50 value of 3.045 μM. In the in vivo antiplasmodial assay, LZ1 exerted a significant antimalarial activity against Plasmodium berghei (P. berghei) in a dose- and a time- dependent manner. In addition, LZ1 exhibited anti-inflammatory effects and attenuated liver-function impairment during P. berghei infection. Furthermore, by employing inhibitors against glycolysis and oxidative phosphorylation in erythrocytes, LZ1 specifically inhibited adenosine triphosphate (ATP) production in parasite-infected erythrocyte by selectively inhibiting the pyruvate kinase activity. In conclusion, the present study demonstrates that LZ1 is a potential candidate for novel antimalarials development.
Keywords: ATP; antimicrobial peptide; cytokine; malaria; pyruvate kinase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
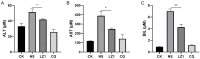
Similar articles
-
Antimalarial efficacy of Pongamia pinnata (L) Pierre against Plasmodium falciparum (3D7 strain) and Plasmodium berghei (ANKA).BMC Complement Altern Med. 2017 Sep 11;17(1):458. doi: 10.1186/s12906-017-1958-y. BMC Complement Altern Med. 2017. Retraction in: BMC Complement Med Ther. 2021 May 10;21(1):139. doi: 10.1186/s12906-021-03312-3. PMID: 28893216 Free PMC article. Retracted.
-
Antiplasmodial efficacy of Calotropis gigantea (L.) against Plasmodium falciparum (3D7 strain) and Plasmodium berghei (ANKA).J Vector Borne Dis. 2017 Jul-Sep;54(3):215-225. doi: 10.4103/0972-9062.217612. J Vector Borne Dis. 2017. PMID: 29097636
-
Evaluation of the antimalarial activity of new compounds against Plasmodium falciparum in vitro, and Plasmodium berghei in vivo.J Pharm Belg. 1990 Sep-Oct;45(5):306-10. J Pharm Belg. 1990. PMID: 2086754
-
Cysteine-stabilised peptide extract of Morinda lucida (Benth) leaf exhibits antimalarial activity and augments antioxidant defense system in P. berghei-infected mice.J Ethnopharmacol. 2017 Jul 31;207:118-128. doi: 10.1016/j.jep.2017.06.026. Epub 2017 Jun 20. J Ethnopharmacol. 2017. PMID: 28645782
-
[Progress of researches on antimalarial peptides].Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2023 Apr 19;35(2):191-198. doi: 10.16250/j.32.1374.2023011. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2023. PMID: 37253570 Review. Chinese.
Cited by
-
Snake Cathelicidin Derived Peptide Inhibits Zika Virus Infection.Front Microbiol. 2020 Aug 4;11:1871. doi: 10.3389/fmicb.2020.01871. eCollection 2020. Front Microbiol. 2020. PMID: 32849457 Free PMC article.
-
Bioactive Peptides against Human Apicomplexan Parasites.Antibiotics (Basel). 2022 Nov 19;11(11):1658. doi: 10.3390/antibiotics11111658. Antibiotics (Basel). 2022. PMID: 36421302 Free PMC article. Review.
-
Aquiluscidin, a Cathelicidin from Crotalus aquilus, and the Vcn-23 Derivative Peptide, Have Anti-Microbial Activity against Gram-Negative and Gram-Positive Bacteria.Microorganisms. 2023 Nov 15;11(11):2778. doi: 10.3390/microorganisms11112778. Microorganisms. 2023. PMID: 38004789 Free PMC article.
-
Animal Venoms and Their Components: Molecular Mechanisms of Action.Toxins (Basel). 2021 Jun 11;13(6):415. doi: 10.3390/toxins13060415. Toxins (Basel). 2021. PMID: 34207957 Free PMC article.
-
Antimalarial Agents as Therapeutic Tools Against Toxoplasmosis-A Short Bridge between Two Distant Illnesses.Molecules. 2020 Mar 30;25(7):1574. doi: 10.3390/molecules25071574. Molecules. 2020. PMID: 32235463 Free PMC article. Review.
References
-
- Global Malaria Programme . World Health Organization; 2018. [(accessed on 30 June 2019)]. World Malaria Report. Available online: https://www.who.int/malaria/publications/world-malaria-report-2018/en/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

