Biosynthetic homeostasis and resilience of the complement system in health and infectious disease
- PMID: 31262714
- PMCID: PMC6642076
- DOI: 10.1016/j.ebiom.2019.06.008
Biosynthetic homeostasis and resilience of the complement system in health and infectious disease
Abstract
Background: The complement system is a central component of the innate immune system. Constitutive biosynthesis of complement proteins is essential for homeostasis. Dysregulation as a consequence of genetic or environmental cues can lead to inflammatory syndromes or increased susceptibility to infection. However, very little is known about steady state levels in children or its kinetics during infection.
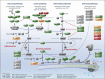
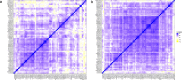
Methods: With a newly developed multiplex mass spectrometry-based method we analyzed the levels of 32 complement proteins in healthy individuals and in a group of pediatric patients infected with bacterial or viral pathogens.
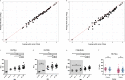
Findings: In plasma from young infants we found reduced levels of C4BP, ficolin-3, factor B, classical pathway components C1QA, C1QB, C1QC, C1R, and terminal pathway components C5, C8, C9, as compared to healthy adults; whereas the majority of complement regulating (inhibitory) proteins reach adult levels at very young age. Both viral and bacterial infections in children generally lead to a slight overall increase in complement levels, with some exceptions. The kinetics of complement levels during invasive bacterial infections only showed minor changes, except for a significant increase and decrease of CRP and clusterin, respectively.
Interpretation: The combination of lower levels of activating and higher levels of regulating complement proteins, would potentially raise the threshold of activation, which might lead to suppressed complement activation in the first phase of life. There is hardly any measurable complement consumption during bacterial or viral infection. Altogether, expression of the complement proteins appears surprisingly stable, which suggests that the system is continuously replenished. FUND: European Union's Horizon 2020, project PERFORM, grant agreement No. 668303.
Keywords: C-reactive protein (CRP); Clusterin; Complement system; Infectious disease; Multiple reaction monitoring (MRM); Targeted mass spectrometry.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Dr. Alkema reports grants from European Commission, during the conduct of the study; Dr. van der Flier reports grants from CSL Behring, grants from Shire, outside the submitted work; Dr. Emonts reports grants from EU FP7, grants from European Union's Horizon 2020 research and innovation programme, during the conduct of the study; personal fees from Newcastle upon Tyne Hospitals NHS Foundation Trust, personal fees from Newcastle University, outside the submitted work; Dr. Irene Rivero-Calle reports other from Ablynx, other from Jansen, other from GSK, other from Medimmune and other from Sanofi Pasteur; personal fees and other from Pfizer, personal fees and other from MSD; all outside the submitted work.
Figures

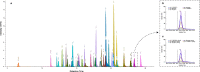


References
-
- Skattum L., van Deuren M., van der Poll T., Truedsson L. Complement deficiency states and associated infections. Mol Immunol. 2011;48(14):1643–1655. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

