CD19 CAR T cells following autologous transplantation in poor-risk relapsed and refractory B-cell non-Hodgkin lymphoma
- PMID: 31262783
- PMCID: PMC6695562
- DOI: 10.1182/blood.2018883421
CD19 CAR T cells following autologous transplantation in poor-risk relapsed and refractory B-cell non-Hodgkin lymphoma
Abstract
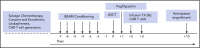
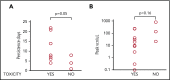
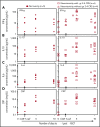
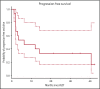
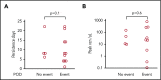
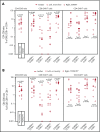
High-dose chemotherapy and autologous stem cell transplantation (HDT-ASCT) is the standard of care for relapsed or primary refractory (rel/ref) chemorefractory diffuse large B-cell lymphoma. Only 50% of patients are cured with this approach. We investigated safety and efficacy of CD19-specific chimeric antigen receptor (CAR) T cells administered following HDT-ASCT. Eligibility for this study includes poor-risk rel/ref aggressive B-cell non-Hodgkin lymphoma chemosensitive to salvage therapy with: (1) positron emission tomography-positive disease or (2) bone marrow involvement. Patients underwent standard HDT-ASCT followed by 19-28z CAR T cells on days +2 and +3. Of 15 subjects treated on study, dose-limiting toxicity was observed at both dose levels (5 × 106 and 1 × 107 19-28z CAR T per kilogram). Ten of 15 subjects experienced CAR T-cell-induced neurotoxicity and/or cytokine release syndrome (CRS), which were associated with greater CAR T-cell persistence (P = .05) but not peak CAR T-cell expansion. Serum interferon-γ elevation (P < .001) and possibly interleukin-10 (P = .07) were associated with toxicity. The 2-year progression-free survival (PFS) is 30% (95% confidence interval, 20% to 70%). Subjects given decreased naive-like (CD45RA+CCR7+) CD4+ and CD8+ CAR T cells experienced superior PFS (P = .02 and .04, respectively). There was no association between CAR T-cell peak expansion, persistence, or cytokine changes and PFS. 19-28z CAR T cells following HDT-ASCT were associated with a high incidence of reversible neurotoxicity and CRS. Following HDT-ASCT, effector CD4+ and CD8+ immunophenotypes may improve disease control. This trial was registered at www.clinicaltrials.gov as #NCT01840566.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: C.S.S., K.J.C., and J.P. have received consulting fees and funding support from Juno Therapeutics, Inc. I.R., M.S., and R.J.B. have received consulting fees and funding support from, and are inventors of patents licensed to, Juno Therapeutics, Inc, in which they have an equity interest. The remaining authors declare no competing financial interests.
Figures


References
-
- Philip T, Guglielmi C, Hagenbeek A, et al. . Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333(23):1540-1545. - PubMed
-
- Vose JM, Carter S, Burns LJ, et al. . Phase III randomized study of rituximab/carmustine, etoposide, cytarabine, and melphalan (BEAM) compared with iodine-131 tositumomab/BEAM with autologous hematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: results from the BMT CTN 0401 trial. J Clin Oncol. 2013;31(13):1662-1668. - PMC - PubMed
-
- Gisselbrecht C, Schmitz N, Mounier N, et al. . Rituximab maintenance therapy after autologous stem-cell transplantation in patients with relapsed CD20(+) diffuse large B-cell lymphoma: final analysis of the collaborative trial in relapsed aggressive lymphoma. J Clin Oncol. 2012;30(36):4462-4469. - PMC - PubMed
-
- Armand P, Welch S, Kim HT, et al. . Prognostic factors for patients with diffuse large B cell lymphoma and transformed indolent lymphoma undergoing autologous stem cell transplantation in the positron emission tomography era. Br J Haematol. 2013;160(5):608-617. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

