AAV2/9-mediated overexpression of MIF inhibits SOD1 misfolding, delays disease onset, and extends survival in mouse models of ALS
- PMID: 31262807
- PMCID: PMC6642395
- DOI: 10.1073/pnas.1904665116
AAV2/9-mediated overexpression of MIF inhibits SOD1 misfolding, delays disease onset, and extends survival in mouse models of ALS
Abstract
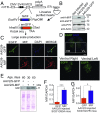
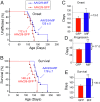
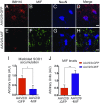
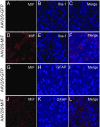
Mutations in superoxide dismutase (SOD1) cause amyotrophic lateral sclerosis (ALS), a neurodegenerative disease characterized by the loss of upper and lower motor neurons. Transgenic mice that overexpress mutant SOD1 develop paralysis and accumulate misfolded SOD1 onto the cytoplasmic faces of intracellular organelles, including mitochondria and endoplasmic reticulum (ER). Recently, macrophage migration inhibitory factor (MIF) was shown to directly inhibit mutant SOD1 misfolding and binding to intracellular membranes. In addition, complete elimination of endogenous MIF accelerated disease onset and late disease progression, as well as shortened the lifespan of mutant SOD1 mice with higher amounts of misfolded SOD1 detected within the spinal cord. Based on these findings, we used adeno-associated viral (AAV) vectors to overexpress MIF in the spinal cord of mutant SOD1G93A and loxSOD1G37R mice. Our data show that MIF mRNA and protein levels were increased in the spinal cords of AAV2/9-MIF-injected mice. Furthermore, mutant SOD1G93A and loxSOD1G37R mice injected with AAV2/9-MIF demonstrated a significant delay in disease onset and prolonged survival compared with their AAV2/9-GFP-injected or noninjected littermates. Moreover, these mice accumulated reduced amounts of misfolded SOD1 in their spinal cords, with no observed effect on glial overactivation as a result of MIF up-regulation. Our findings indicate that MIF plays a significant role in SOD1 folding and misfolding mechanisms and strengthen the hypothesis that MIF acts as a chaperone for misfolded SOD1 in vivo and may have further implications regarding the therapeutic potential role of up-regulation of MIF in modulating the specific accumulation of misfolded SOD1.
Keywords: AAV; ALS; MIF; misfolded SOD1; mutant SOD1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Endogenous macrophage migration inhibitory factor reduces the accumulation and toxicity of misfolded SOD1 in a mouse model of ALS.Proc Natl Acad Sci U S A. 2016 Sep 6;113(36):10198-203. doi: 10.1073/pnas.1604600113. Epub 2016 Aug 22. Proc Natl Acad Sci U S A. 2016. PMID: 27551074 Free PMC article.
-
Targeting low levels of MIF expression as a potential therapeutic strategy for ALS.Cell Rep Med. 2024 May 21;5(5):101546. doi: 10.1016/j.xcrm.2024.101546. Epub 2024 May 3. Cell Rep Med. 2024. PMID: 38703766 Free PMC article.
-
Macrophage migration inhibitory factor as a chaperone inhibiting accumulation of misfolded SOD1.Neuron. 2015 Apr 8;86(1):218-32. doi: 10.1016/j.neuron.2015.02.034. Epub 2015 Mar 19. Neuron. 2015. PMID: 25801706 Free PMC article.
-
Does wild-type Cu/Zn-superoxide dismutase have pathogenic roles in amyotrophic lateral sclerosis?Transl Neurodegener. 2020 Aug 19;9(1):33. doi: 10.1186/s40035-020-00209-y. Transl Neurodegener. 2020. PMID: 32811540 Free PMC article. Review.
-
Transgenic mice with human mutant genes causing Parkinson's disease and amyotrophic lateral sclerosis provide common insight into mechanisms of motor neuron selective vulnerability to degeneration.Rev Neurosci. 2007;18(2):115-36. doi: 10.1515/revneuro.2007.18.2.115. Rev Neurosci. 2007. PMID: 17593875 Review.
Cited by
-
A mutation in the PRKAR1B gene drives pathological mechanisms of neurodegeneration across species.Brain. 2024 Nov 4;147(11):3890-3905. doi: 10.1093/brain/awae154. Brain. 2024. PMID: 38743596 Free PMC article.
-
Molecular and pharmacological chaperones for SOD1.Biochem Soc Trans. 2020 Aug 28;48(4):1795-1806. doi: 10.1042/BST20200318. Biochem Soc Trans. 2020. PMID: 32794552 Free PMC article. Review.
-
The Potential Role of Cytokines and Growth Factors in the Pathogenesis of Alzheimer's Disease.Cells. 2021 Oct 18;10(10):2790. doi: 10.3390/cells10102790. Cells. 2021. PMID: 34685770 Free PMC article. Review.
-
BMP9 reduces age-related bone loss in mice by inhibiting osteoblast senescence through Smad1-Stat1-P21 axis.Cell Death Discov. 2022 May 6;8(1):254. doi: 10.1038/s41420-022-01048-8. Cell Death Discov. 2022. PMID: 35523787 Free PMC article.
-
4-Phenylbutyric Acid (4-PBA) Derivatives Prevent SOD1 Amyloid Aggregation In Vitro with No Effect on Disease Progression in SOD1-ALS Mice.Int J Mol Sci. 2022 Aug 20;23(16):9403. doi: 10.3390/ijms23169403. Int J Mol Sci. 2022. PMID: 36012668 Free PMC article.
References
-
- Rosen D. R., et al. , Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 (1993). - PubMed
-
- Turner B. J., Talbot K., Transgenics, toxicity and therapeutics in rodent models of mutant SOD1-mediated familial ALS. Prog. Neurobiol. 85, 94–134 (2008). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

