Impact of audit and feedback with action implementation toolbox on improving ICU pain management: cluster-randomised controlled trial
- PMID: 31263017
- PMCID: PMC6934240
- DOI: 10.1136/bmjqs-2019-009588
Impact of audit and feedback with action implementation toolbox on improving ICU pain management: cluster-randomised controlled trial
Abstract
Background: Audit and feedback (A&F) enjoys widespread use, but often achieves only marginal improvements in care. Providing recipients of A&F with suggested actions to overcome barriers (action implementation toolbox) may increase effectiveness.
Objective: To assess the impact of adding an action implementation toolbox to an electronic A&F intervention targeting quality of pain management in intensive care units (ICUs).
Trial design: Two-armed cluster-randomised controlled trial. Randomisation was computer generated, with allocation concealment by a researcher, unaffiliated with the study. Investigators were not blinded to the group assignment of an ICU.
Participants: Twenty-one Dutch ICUs and patients eligible for pain measurement.
Interventions: Feedback-only versus feedback with action implementation toolbox.
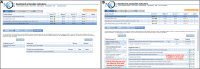
Outcome: Proportion of patient-shift observations where pain management was adequate; composed by two process (measuring pain at least once per patient in each shift; re-measuring unacceptable pain scores within 1 hour) and two outcome indicators (acceptable pain scores; unacceptable pain scores normalised within 1 hour).
Results: 21 ICUs (feedback-only n=11; feedback-with-toolbox n=10) with a total of 253 530 patient-shift observations were analysed. We found absolute improvement on adequate pain management in the feedback-with-toolbox group (14.8%; 95% CI 14.0% to 15.5%) and the feedback-only group (4.8%; 95% CI 4.2% to 5.5%). Improvement was limited to the two process indicators. The feedback-with-toolbox group achieved larger effects than the feedback-only group both on the composite adequate pain management (p<0.05) and on measuring pain each shift (p<0.001). No important adverse effects have occurred.
Conclusion: Feedback with toolbox improved the number of shifts where patients received adequate pain management compared with feedback alone, but only in process and not outcome indicators.
Trial registration number: NCT02922101.
Keywords: action implementation toolbox; dashboard; feedback; intensive care units; pain; quality improvement.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: The National Intensive Care Evaluation (NICE) Foundation pays the Department of Medical Informatics, Academic Medical Center for processing, checking and maintaining the Dutch quality registry. M-JR-B, WTG and NFdK are employees of the Department of Medical Informatics and work for the NICE registry. NFdK, DAD, EdJ and JJS are members of the NICE board.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
