DNA double-strand break repair-pathway choice in somatic mammalian cells
- PMID: 31263220
- PMCID: PMC7315405
- DOI: 10.1038/s41580-019-0152-0
DNA double-strand break repair-pathway choice in somatic mammalian cells
Abstract
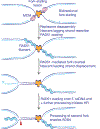
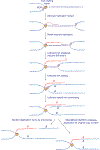
The major pathways of DNA double-strand break (DSB) repair are crucial for maintaining genomic stability. However, if deployed in an inappropriate cellular context, these same repair functions can mediate chromosome rearrangements that underlie various human diseases, ranging from developmental disorders to cancer. The two major mechanisms of DSB repair in mammalian cells are non-homologous end joining (NHEJ) and homologous recombination. In this Review, we consider DSB repair-pathway choice in somatic mammalian cells as a series of 'decision trees', and explore how defective pathway choice can lead to genomic instability. Stalled, collapsed or broken DNA replication forks present a distinctive challenge to the DSB repair system. Emerging evidence suggests that the 'rules' governing repair-pathway choice at stalled replication forks differ from those at replication-independent DSBs.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures




References
-
- Sung P & Klein H Mechanism of homologous recombination: mediators and helicases take on regulatory functions. Nat Rev Mol Cell Biol 7, 739–750 (2006). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

