Phosphorylated Rho-GDP directly activates mTORC2 kinase towards AKT through dimerization with Ras-GTP to regulate cell migration
- PMID: 31263268
- PMCID: PMC6650273
- DOI: 10.1038/s41556-019-0348-8
Phosphorylated Rho-GDP directly activates mTORC2 kinase towards AKT through dimerization with Ras-GTP to regulate cell migration
Abstract
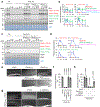
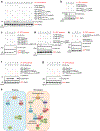
mTORC2 plays critical roles in metabolism, cell survival and actin cytoskeletal dynamics through the phosphorylation of AKT. Despite its importance to biology and medicine, it is unclear how mTORC2-mediated AKT phosphorylation is controlled. Here, we identify an unforeseen principle by which a GDP-bound form of the conserved small G protein Rho GTPase directly activates mTORC2 in AKT phosphorylation in social amoebae (Dictyostelium discoideum) cells. Using biochemical reconstitution with purified proteins, we demonstrate that Rho-GDP promotes AKT phosphorylation by assembling a supercomplex with Ras-GTP and mTORC2. This supercomplex formation is controlled by the chemoattractant-induced phosphorylation of Rho-GDP at S192 by GSK-3. Furthermore, Rho-GDP rescues defects in both mTORC2-mediated AKT phosphorylation and directed cell migration in Rho-null cells in a manner dependent on phosphorylation of S192. Thus, in contrast to the prevailing view that the GDP-bound forms of G proteins are inactive, our study reveals that mTORC2-AKT signalling is activated by Rho-GDP.
Figures





Similar articles
-
KARATE: PKA-induced KRAS4B-RHOA-mTORC2 supercomplex phosphorylates AKT in insulin signaling and glucose homeostasis.Mol Cell. 2021 Nov 18;81(22):4622-4634.e8. doi: 10.1016/j.molcel.2021.09.001. Epub 2021 Sep 21. Mol Cell. 2021. PMID: 34551282
-
Hetero-oligomerization of Rho and Ras GTPases Connects GPCR Activation to mTORC2-AKT Signaling.Cell Rep. 2020 Nov 24;33(8):108427. doi: 10.1016/j.celrep.2020.108427. Cell Rep. 2020. PMID: 33238110 Free PMC article.
-
Stimulation of mTORC2 by integrin αIIbβ3 is required for PI3Kβ-dependent activation of Akt but is dispensable for platelet spreading on fibrinogen.Platelets. 2020 May 18;31(4):521-529. doi: 10.1080/09537104.2019.1663806. Epub 2019 Sep 11. Platelets. 2020. PMID: 31509054
-
Regulation of phosphorylation pathways by p21 GTPases. The p21 Ras-related Rho subfamily and its role in phosphorylation signalling pathways.Eur J Biochem. 1996 Dec 1;242(2):171-85. doi: 10.1111/j.1432-1033.1996.0171r.x. Eur J Biochem. 1996. PMID: 8973630 Review.
-
Ras, PI3K and mTORC2 - three's a crowd?J Cell Sci. 2020 Oct 8;133(19):jcs234930. doi: 10.1242/jcs.234930. J Cell Sci. 2020. PMID: 33033115 Free PMC article. Review.
Cited by
-
Active GTPase Pulldown Protocol.Methods Mol Biol. 2021;2262:117-135. doi: 10.1007/978-1-0716-1190-6_7. Methods Mol Biol. 2021. PMID: 33977474
-
Using Drosophila melanogaster to Dissect the Roles of the mTOR Signaling Pathway in Cell Growth.Cells. 2023 Nov 14;12(22):2622. doi: 10.3390/cells12222622. Cells. 2023. PMID: 37998357 Free PMC article. Review.
-
Emerging Roles for Mammalian Target of Rapamycin (mTOR) Complexes in Bladder Cancer Progression and Therapy.Cancers (Basel). 2022 Mar 18;14(6):1555. doi: 10.3390/cancers14061555. Cancers (Basel). 2022. PMID: 35326708 Free PMC article. Review.
-
CRISPR/Cas9-based genome-wide screening of Dictyostelium.Sci Rep. 2022 Jul 2;12(1):11215. doi: 10.1038/s41598-022-15500-3. Sci Rep. 2022. PMID: 35780186 Free PMC article.
-
Rho-GTPases subfamily: cellular defectors orchestrating viral infection.Cell Mol Biol Lett. 2025 May 2;30(1):55. doi: 10.1186/s11658-025-00722-w. Cell Mol Biol Lett. 2025. PMID: 40316910 Free PMC article. Review.
References
-
- Hodge RG & Ridley AJ Regulating Rho GTPases and their regulators. Nat Rev Mol Cell Biol 17, 496–510 (2016). - PubMed
-
- Burridge K & Wennerberg K Rho and Rac take center stage. Cell 116, 167–179 (2004). - PubMed
-
- Stenmark H Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10, 513–525 (2009). - PubMed
-
- Alberts B et al. Molecular Biology of the Cell, Sixth Edition Molecular Biology of the Cell, Sixth Edition, 1–1342 (2015).
-
- Mayor R & Etienne-Manneville S The front and rear of collective cell migration. Nat Rev Mol Cell Biol 17, 97–109 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

