A Novel Antiplatelet Aggregation Target of Justicidin B Obtained From Rostellularia Procumbens (L.) Nees
- PMID: 31263419
- PMCID: PMC6590258
- DOI: 10.3389/fphar.2019.00688
A Novel Antiplatelet Aggregation Target of Justicidin B Obtained From Rostellularia Procumbens (L.) Nees
Abstract
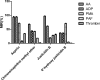
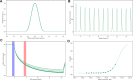
The present study explored the possible bioactive ingredients and target protein of Rostellularia procumbens (L.) Nees. Firstly, we found that the ethyl acetate extraction obtained from R. procumbens could inhibit platelet aggregation. Then, gene chip was used to investigate differentially expressed genes and blood absorption compounds were investigated using high performance liquid chromatography-mass spectrometry characterization (LC-MS). Depending on the results of gene chip and LC-MS, the targets of blood absorption compounds were predicted according to the reverse pharmacophore matching model. The platelet aggregation-related genes were discovered in databases, and antiplatelet aggregation-related gene targets were selected through comparison. The functions of target genes and related pathways were analyzed and screened using the DAVID database, and the network was constructed using Cytoscape software. We found that integrin αIIbβ3 had a highest degree, and it was almost the intersection of all pathways. Then, blood absorption compounds were screened by optical turbidimetry. Western blot (WB) revealed that justicidin B separated from the ethyl acetate fraction may inhibit the expression of integrin αIIbβ3 protein. For the first time, we used Prometheus NT.48 and MST to detect the stability of this membrane protein to optimize the buffer and studied the interaction of justicidin B with its target protein. To our best knowledge, this is the first report to state that justicidin B targets the integrin αIIbβ3 protein. We believe that our findings can provide a novel target protein for the further understanding of the mechanism of R. procumbens on platelet aggregation.
Keywords: LC-MS; Prometheus NT.48; gene chip; integrin αIIbβ3; justicidin B; microscale thermophoresis; network pharmacology; platelet aggregation.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials

