The PD-1/PD-L1 Axis and Virus Infections: A Delicate Balance
- PMID: 31263684
- PMCID: PMC6584848
- DOI: 10.3389/fcimb.2019.00207
The PD-1/PD-L1 Axis and Virus Infections: A Delicate Balance
Abstract
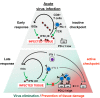
Programmed cell death protein (PD-1) and its ligands play a fundamental role in the evasion of tumor cells from antitumor immunity. Less well appreciated is the fact that the PD-1/PD-L1 axis also regulates antiviral immune responses and is therefore modulated by a number of viruses. Upregulation of PD-1 and its ligands PD-L1 and PD-L2 is observed during acute virus infection and after infection with persistent viruses including important human pathogens such as human immunodeficiency virus (HIV), hepatitis C virus (HCV), and hepatitis B virus (HBV). Experimental evidence suggests that insufficient signaling through the PD-1 pathway promotes immunopathology during acute infection by exaggerating primary T cell responses. If chronic infection is established, however, high levels of PD-1 expression can have unfavorable immunological consequences. Exhaustion and suppression of antiviral immune responses can result in viral immune evasion. The role of the PD-1/PD-L1 axis during viral infections is further complicated by evidence that PD-L1 also mediates inflammatory effects in the acute phase of an immune response. In this review, we discuss the intricate interplay between viruses and the PD-1/PD-L1 axis.
Keywords: PD-1; PD-L1; PD-L2; antiviral immune responses; viral immune evasion; virus-induced immunopathogenesis; viruses.
Figures

References
-
- Agata Y., Kawasaki A., Nishimura H., Ishida Y., Tsubata T., Yagita H., et al. . (1996). Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 8, 765–772. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

