Golgi pH, Ion and Redox Homeostasis: How Much Do They Really Matter?
- PMID: 31263697
- PMCID: PMC6584808
- DOI: 10.3389/fcell.2019.00093
Golgi pH, Ion and Redox Homeostasis: How Much Do They Really Matter?
Abstract
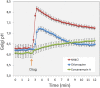
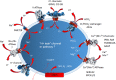
Exocytic and endocytic compartments each have their own unique luminal ion and pH environment that is important for their normal functioning. A failure to maintain this environment - the loss of homeostasis - is not uncommon. In the worst case, all the main Golgi functions, including glycosylation, membrane trafficking and protein sorting, can be perturbed. Several factors contribute to Golgi homeostasis. These include not only ions such as H+, Ca2+, Mg2+, Mn2+, but also Golgi redox state and nitric oxide (NO) levels, both of which are dependent on the oxygen levels in the cells. Changes to any one of these factors have consequences on Golgi functions, the nature of which can be dissimilar or similar depending upon the defects themselves. For example, altered Golgi pH homeostasis gives rise to Cutis laxa disease, in which glycosylation and membrane trafficking are both affected, while altered Ca2+ homeostasis due to the mutated SCPA1 gene in Hailey-Hailey disease, perturbs various protein sorting, proteolytic cleavage and membrane trafficking events in the Golgi. This review gives an overview of the molecular machineries involved in the maintenance of Golgi ion, pH and redox homeostasis, followed by a discussion of the organelle dysfunction and disease that frequently result from their breakdown. Congenital disorders of glycosylation (CDGs) are discussed only when they contribute directly to Golgi pH, ion or redox homeostasis. Current evidence emphasizes that, rather than being mere supporting factors, Golgi pH, ion and redox homeostasis are in fact key players that orchestrate and maintain all Golgi functions.
Keywords: Golgi pH; Golgi redox state; cancer; glycosylation; homeostasis; protein sorting.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

