Spontaneous Formation of CdSe Photoluminescent Nanotubes with Visible-Light Photocatalytic Performance
- PMID: 31263761
- PMCID: PMC6598157
- DOI: 10.1021/acscentsci.9b00184
Spontaneous Formation of CdSe Photoluminescent Nanotubes with Visible-Light Photocatalytic Performance
Abstract
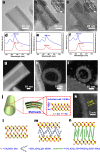
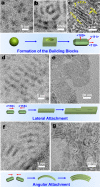
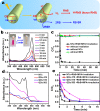
Two-dimensional (2D) colloidal CdSe nanocrystals (NCs) with precise atomic-scale thickness have attracted intensive attention in recent years due to their optical properties and quantum confinement effects originating from their particular band structure. Here, we report a solution-based and template-free protocol to synthesize CdSe nanotubes (NTs) having 3-6 walls, each of which has 3.5 molecular monolayers. Their crystal structure is zincblende, with Cd-terminated {100} planes at the top and bottom surfaces of each wall, which are passivated by short-chain acetate ligands. After verifying the prominent role of the acetate ligand for NT synthesis, we elucidated the formation mechanism of these NTs. It starts by heterogeneous nucleation of 2D plateletlike nanoseeds from the amorphous Cd precursor matrix, followed by the growth via lateral and angular attachment of nanoplatelet building blocks into curved nanosheets, eventually resulting in NTs with sharp absorption and photoluminescence peak at around 460 nm. Moreover, the NTs show remarkable visible-light photocatalytic activity, as demonstrated by the reduction of the reddish Rhodamine B into its leuco form with a conversion rate of 92% in 1 min.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials

