Dimethyl fumarate abrogates dust mite-induced allergic asthma by altering dendritic cell function
- PMID: 31264384
- PMCID: PMC6688084
- DOI: 10.1002/iid3.262
Dimethyl fumarate abrogates dust mite-induced allergic asthma by altering dendritic cell function
Abstract
Introduction: Allergic asthma is the most common inflammatory disease of upper airways. Airway dendritic cells (DCs) are key antigen presenting cells that regulate T helper 2 (Th2)-dependent allergic inflammation. Recent studies have shown critical role of airway DCs in the induction of Th2-mediated allergic inflammation and are attractive therapeutic targets in asthma. However, molecular signaling mechanism that regulate DCs function to Th2 immune responses are poorly understood. Here we aim to evaluate the immunomodulatory effect of dimethyl fumarate (DMF), an FDA approved small molecule drug, in the house dust mite (HDM)-induced experimental model of allergic asthma.
Methods: DMF was administered intranasally in the challenge period of HDM-induced murine model of experimental asthma. Airway inflammation, airway hyperreactivity, Th2/Th1 cytokine were assessed. The effect of DMF on DC function was further evaluated by adoptive transfer of HDM-pulsed DMF treated DCs to wild-type naïve mice.
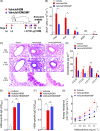
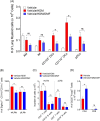
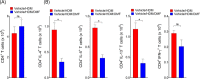
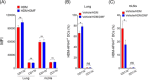
Results: DMF treatment significantly reduced HDM-induced airway inflammation, mucous cell metaplasia, and airway hyperactivity to inhaled methacholine. Mechanistically, DMF interferes with the migration of lung DCs to draining mediastinal lymph nodes, thereby attenuates the induction of allergic sensitization and Th2 immune response. Notably, adoptive transfer of DMF treated DCs to naïve mice with HDM challenge similarly reduces the features of allergic asthma.
Conclusion: This identifies a novel function of DMF on DC-mediated adaptive immune responses in the setting of HDM-induced airway inflammation. Taken together, our results offer a mechanistic rationale for DMF use to target DCs in local lung environment as antiasthmatic therapy.
Keywords: allergic asthma; dendritic cells; dimethyl fumarate; house dust mite.
© 2019 The Authors. Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures

Similar articles
-
Dectin-2 promotes house dust mite-induced T helper type 2 and type 17 cell differentiation and allergic airway inflammation in mice.Am J Respir Cell Mol Biol. 2014 Aug;51(2):201-9. doi: 10.1165/rcmb.2013-0522OC. Am J Respir Cell Mol Biol. 2014. PMID: 24588637
-
Notch signaling in T cells is essential for allergic airway inflammation, but expression of the Notch ligands Jagged 1 and Jagged 2 on dendritic cells is dispensable.J Allergy Clin Immunol. 2017 Oct;140(4):1079-1089. doi: 10.1016/j.jaci.2016.11.046. Epub 2017 Jan 19. J Allergy Clin Immunol. 2017. PMID: 28111308
-
Tilianin attenuates HDM-induced allergic asthma by suppressing Th2-immune responses via downregulation of IRF4 in dendritic cells.Phytomedicine. 2021 Jan;80:153392. doi: 10.1016/j.phymed.2020.153392. Epub 2020 Oct 20. Phytomedicine. 2021. PMID: 33113503
-
Modeling responses to respiratory house dust mite exposure.Contrib Microbiol. 2007;14:42-67. doi: 10.1159/000107054. Contrib Microbiol. 2007. PMID: 17684332 Review.
-
Dendritic Cells: Critical Regulators of Allergic Asthma.Int J Mol Sci. 2020 Oct 26;21(21):7930. doi: 10.3390/ijms21217930. Int J Mol Sci. 2020. PMID: 33114551 Free PMC article. Review.
Cited by
-
Irg1/itaconate metabolic pathway is a crucial determinant of dendritic cells immune-priming function and contributes to resolute allergen-induced airway inflammation.Mucosal Immunol. 2022 Feb;15(2):301-313. doi: 10.1038/s41385-021-00462-y. Epub 2021 Oct 20. Mucosal Immunol. 2022. PMID: 34671116 Free PMC article.
-
Calming the (Cytokine) Storm: Dimethyl Fumarate as a Therapeutic Candidate for COVID-19.Pharmaceuticals (Basel). 2020 Dec 26;14(1):15. doi: 10.3390/ph14010015. Pharmaceuticals (Basel). 2020. PMID: 33375288 Free PMC article.
-
The role of DC subgroups in the pathogenesis of asthma.Front Immunol. 2024 Oct 28;15:1481989. doi: 10.3389/fimmu.2024.1481989. eCollection 2024. Front Immunol. 2024. PMID: 39530090 Free PMC article. Review.
-
Lipoxin A4 yields an electrophilic 15-oxo metabolite that mediates FPR2 receptor-independent anti-inflammatory signaling.J Lipid Res. 2025 Jan;66(1):100705. doi: 10.1016/j.jlr.2024.100705. Epub 2024 Nov 19. J Lipid Res. 2025. PMID: 39566850 Free PMC article.
-
Novel potential pharmacological applications of dimethyl fumarate-an overview and update.Front Pharmacol. 2023 Sep 7;14:1264842. doi: 10.3389/fphar.2023.1264842. eCollection 2023. Front Pharmacol. 2023. PMID: 37745068 Free PMC article. Review.
References
-
- Lehmann JC, Listopad JJ, Rentzsch CU, et al. Dimethylfumarate induces immunosuppression via glutathione depletion and subsequent induction of heme oxygenase 1. J Invest Dermatol. 2007;127:835‐845. - PubMed
-
- Gold R, Kappos L, Arnold DL. Placebo‐controlled phase 3 study of oral BG‐12 for relapsing multiple sclerosis. New Engl J Med. 2012;367:1098. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

