Use of sodium-glucose co-transporter-2 inhibitors in patients with type 2 diabetes mellitus and multiple cardiovascular risk factors: An Asian perspective and expert recommendations
- PMID: 31264765
- PMCID: PMC6852284
- DOI: 10.1111/dom.13819
Use of sodium-glucose co-transporter-2 inhibitors in patients with type 2 diabetes mellitus and multiple cardiovascular risk factors: An Asian perspective and expert recommendations
Abstract
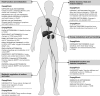
Diabetes mellitus in Asia accounts for more than half of the global prevalence. There is a high prevalence of cardiovascular disease (CVD) in the region among people with type 2 diabetes mellitus (T2DM) and it is often associated with multiple risk factors including hypertension, renal disease and obesity. The early onset of T2DM and the eventual long disease duration portends an increasing proportion of the population to premature CVD. In addition to lowering blood glucose, sodium-glucose co-transporter-2 (SGLT-2) inhibitors exert favourable effects on multiple risk factors (including blood pressure, body weight and renal function) and provide an opportunity to reduce the risk of CVD in patients with T2DM. In this article, we consolidated the existing literature on SGLT-2 inhibitor use in Asian patients with T2DM and established contemporary guidance for clinicians. We extensively reviewed recommendations from international and regional guidelines, published data from clinical trials in the Asian population (dapagliflozin, canagliflozin, empagliflozin, ipragliflozin, luseogliflozin and tofogliflozin), CVD outcomes trials (EMPAREG-OUTCOME, CANVAS and DECLARE-TIMI 58) and real-world evidence studies (CVD-REAL, EASEL, CVD-REAL 2 and OBSERVE-4D). A series of clinical recommendations on the use of SGLT-2 inhibitors in Asian patients with T2DM was deliberated among experts with multiple rounds of review and voting. Based on the available evidence, we conclude that SGLT-2 inhibitors represent an evidence-based therapeutic option for the primary prevention of heart failure hospitalization and secondary prevention of CVD in patients with T2DM, and should be considered early on in the treatment algorithm for patients with multiple risk factors, or those with established CVD.
Keywords: cardiovascular; diabetes; gliflozins; sodium-glucose co-transporter-2 inhibitors; type 2 diabetes mellitus.
© 2019 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
C.D. has received honoraria as the speaker or advisor or research grant from AstraZeneca, Boehringer Ingelheim, Janssen, Bayer, Eli Lilly, Abbott, Novartis, Pfizer, Merck Sharp & Dohme, Novo Nordisk, Sanofi and Takeda. S.P.C. has received honoraria as speaker and advisor for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Merck Sharp & Dohme, Novartis, Novo Nordisk, Sanofi and Servier. B.J.M. has received honoraria and CME grants from AstraZeneca, Boehringer Ingelheim, Lilly, Merck, Multicare, MSD, NatraPharm, Novartis, Novo Nordisk, Pfizer, Sanofi, Servier and Torrent Pharma. W.H.‐H.S. has been an advisor and/or speaker for AstraZeneca, Bayer HealthCare, Boehringer Ingelheim Pharmaceuticals, Daiichi‐Sankyo, Eli Lilly and Company, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma Corporation, Novartis Pharmaceuticals, Novo Nordisk, Pfizer, Sanofi‐Aventis and Takeda Pharmaceutical Company. J.C. is the Chief Executive Officer (on pro‐bono basis) of Asia Diabetes Foundation, a charitable foundation established under The Chinese University of Hong Kong Foundation for developing the JADE Technology; she has received honoraria and travelling support for consultancy or giving lectures and her affiliated institutions have received research and educational grants from Amgen, Ascencia, AstraZeneca, Bayer, Bristol‐Myers Squibb, Boehringer Ingelheim, Daiichi‐Sankyo, Eli‐Lilly, GlaxoSmithKline, Medtronic, Merck Serono, Merck Sharp & Dohme, Novo Nordisk, Pfizer and Sanofi. N.H.M. has no conflict of interest to declare. K.S. has received honoraria as a speaker or advisor from AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme, Novo Nordisk, Sanofi, Merck and Servier. A.L. is a member of advisory boards for AstraZeneca, Boehringer Ingelheim, Sanofi and Amgen; she has received research grants from Boehringer Ingelheim, MSD, Sanofi and Amgen, and travel grants from AstraZeneca, Boehringer Ingelheim, MSD, Novartis, Novo Nordisk and Sanofi. C.M.K. has received honoraria as a speaker or advisor from AstraZeneca, Boehringer Ingelheim, Novo Nordisk and Sanofi. K.‐H.Y. has received honoraria as a speaker or advisor from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceutical, Merck Sharp & Dohme, Novo Nordisk, Sanofi and Takeda, and research support from AstraZeneca and Takeda. A.M. has received honoraria as a speaker and advisor from AstraZeneca, Abbott, Boehringer Ingelheim, Cipla, Dr. Reddy's, Eli Lilly, Glenmark, Glaxosmithkline, Ipca, Janssen, Lupin, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, Sanofi, Serdia Servier, Sun Pharma, Torrent, Wockhardt and Zydus Nutrition. J.L. is a member of the DISCOVER Scientific Committee and received support from AstraZeneca to attend DISCOVER planning and update meetings; he has also received honoraria from Eli Lilly, Bristol‐Myers Squibb, Novartis, Novo Nordisk, Bayer, Merck Sharp & Dohme, Takeda, Sanofi, Roche, Boehringer Ingelheim and AstraZeneca, and research support from Roche, Sanofi, Merck Sharp & Dohme, AstraZeneca, Novartis, Eli Lilly and Bristol‐Myers Squibb.
Figures

References
-
- WHO . The top 10 causes of death. http://www.who.int/mediacentre/factsheets/fs310/en/. Updated January 2017. Accessed April 2, 2019.
-
- World Health Organization . Noncommunicable diseases. https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases. Updated June 2018. Accessed April 2, 2019.
-
- White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327‐1335. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

