Brain circuitry, behavior, and cognition: A randomized placebo-controlled trial of donepezil in fragile X syndrome
- PMID: 31264943
- PMCID: PMC6894490
- DOI: 10.1177/0269881119858304
Brain circuitry, behavior, and cognition: A randomized placebo-controlled trial of donepezil in fragile X syndrome
Abstract
Background: Fragile X syndrome, the most common inherited cause for intellectual disability, is associated with alterations in cholinergic among other neurotransmitter systems. This study investigated the effects of donepezil hydrochloride, a cholinesterase inhibitor that has potential to correct aberrant cholinergic signaling.
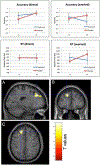
Method: Forty-two individuals with fragile X syndrome (mean age=19.61 years) were randomized to receive 2.5-10.0 mg of donepezil (n=20, seven females) or placebo (n=22, eight females) per day. One individual in the active group withdrew at week 7. Outcomes included the contingency naming test, the aberrant behavior checklist, and behavior and brain activation patterns during a functional magnetic resonance imaging gaze discrimination task.
Results: There were no significant differences between active and placebo groups on cognitive (contingency naming task) or behavioral (total score or subscales of the aberrant behavior checklist) outcomes. At baseline, the active and placebo groups did not differ in functional magnetic resonance imaging activation patterns during the gaze task. After 12 weeks of treatment the active group displayed reduced activation in response to the averted vs direct gaze contrast, relative to the placebo group, in the left superior frontal gyrus.
Conclusions: Reduced functional brain activation for the active group may represent less arousal in response to direct eye gaze, relative to the placebo group. Change in functional magnetic resonance imaging activation patterns may serve as a more sensitive metric and predictor of response to treatment when compared to cognitive and behavioral assessments. Our results suggest that donepezil may have an impact on brain functioning, but longer term follow-up and concomitant behavioral intervention may be required to demonstrate improvement in cognition and behavior.
Keywords: Clinical trial; autism spectrum disorder; fragile X syndrome; functional magnetic resonance imaging; neuroimaging.
Conflict of interest statement
Declaration of Interest: Dr. Bruno reports consulting for Balance Pharmaceuticals regarding clinical trial data for research in Down syndrome. The remaining authors report no conflicts of interest.
Figures


References
-
- Aman MG, Burrow WH and Wolford PL (1995) The Aberrant Behavior Checklist-Community - Factor Validity and Effect of Subject Variables for Adults in Group Homes. American Journal on Mental Retardation 100(3): 283–292. - PubMed
-
- Anderson P, Anderson V, Northam E, et al. (2000) Standardization of the Contingency Naming Test (CNT) for school-aged children: A measure of reactive flexibility. Clinical Neuropsychological Assessment 1: 247–273.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

