Validation of the Seegene RV15 multiplex PCR for the detection of influenza A subtypes and influenza B lineages during national influenza surveillance in hospitalized adults
- PMID: 31264957
- PMCID: PMC7431100
- DOI: 10.1099/jmm.0.001032
Validation of the Seegene RV15 multiplex PCR for the detection of influenza A subtypes and influenza B lineages during national influenza surveillance in hospitalized adults
Abstract
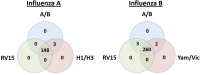
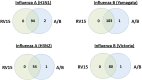
Background. The Serious Outcomes Surveillance Network of the Canadian Immunization Research Network (CIRN SOS) has been performing active influenza surveillance since 2009 (ClinicalTrials.gov identifier: NCT01517191). Influenza A and B viruses are identified and characterized using real-time reverse-transcriptase polymerase chain reaction (RT-PCR), and multiplex testing has been performed on a subset of patients to identify other respiratory virus aetiologies. Since both methods can identify influenza A and B, a direct comparison was performed.Methods. Validated real-time RT-PCRs from the World Health Organization (WHO) to identify influenza A and B viruses, characterize influenza A viruses into the H1N1 or H3N2 subtypes and describe influenza B viruses belonging to the Yamagata or Victoria lineages. In a subset of patients, the Seeplex RV15 One-Step ACE Detection assay (RV15) kit was also used for the detection of other respiratory viruses.Results. In total, 1111 nasopharyngeal swabs were tested by RV15 and real-time RT-PCRs for influenza A and B identification and characterization. For influenza A, RV15 showed 98.0 % sensitivity, 100 % specificity and 99.7 % accuracy. The performance characteristics of RV15 were similar for influenza A subtypes H1N1 and H3N2. For influenza B, RV15 had 99.2 % sensitivity, 100 % specificity and 99.8 % accuracy, with similar assay performance being shown for both the Yamagata and Victoria lineages.Conclusions. Overall, the detection of circulating subtypes of influenza A and lineages of influenza B by RV15 was similar to detection by real-time RT-PCR. Multiplex testing with RV15 allows for a more comprehensive respiratory virus surveillance in hospitalized adults, without significantly compromising the reliability of influenza A or B virus detection.
Keywords: influenza; lineage; multiplex PCR; subtype; validation.
Conflict of interest statement
J. L. received research grants from Merck for work outside the study, but no personal payments. T. F. H. and S. A. M. report payments to their institution from the GSK group of companies for the conduct of the study, and payments from Pfizer, Merck, Novartis and Sanofi‐Pasteur outside the submitted work. M. K. A. reports grants from the GSK group of companies, Pfizer and Sanofi, but no personal payments. J. M. reports payments to her institution from the GSK group of companies and Sanofi for her participation in advisory boards. A. P. reports payments from Actelion, Sanofi‐Pasteur and Genentech. J. P. reports payments from the GSK group of companies, Merck, Roche and Synthetic Biologics outside the submitted work. L. V. received research grants from the GSK group of companies, Pfizer, Optimer, Cubist and Merck, and personal fees from Merck, Optimer and Cubist. G. D. S. reports he was external consultant at Business and Decision Life Sciences (on behalf of GSK) at the time of the study, and is currently employed by the GSK group of companies and holds shares in the GSK group of companies. No other conflicts were declared.
Figures
References
-
- McNeil S, Shinde V, Andrew M, Hatchette T, Leblanc J, et al. Interim estimates of 2013/14 influenza clinical severity and vaccine effectiveness in the prevention of laboratory-confirmed influenza-related hospitalisation, Canada, February 2014. Euro Surveill. 2014;19:20729. doi: 10.2807/1560-7917.ES2014.19.9.20729. - DOI - PubMed
-
- McNeil SA, Andrew MK, Ye L, Haguinet F, Hatchette TF, et al. Interim estimates of 2014/15 influenza vaccine effectiveness in preventing laboratory-confirmed influenza-related hospitalisation from the Serious Outcomes Surveillance Network of the Canadian Immunization Research Network, January 2015. Euro Surveill. 2015;20:21024. doi: 10.2807/1560-7917.ES2015.20.5.21024. - DOI - PubMed
-
- Andrew MK, Shinde V, Hatchette T, Ambrose A, Boivin G, et al. Influenza vaccine effectiveness against influenza-related hospitalization during a season with mixed outbreaks of four influenza viruses: a test-negative case-control study in adults in Canada. BMC Infect Dis. 2017;17:805. doi: 10.1186/s12879-017-2905-8. - DOI - PMC - PubMed
-
- Nichols MK, Andrew MK, Hatchette TF, Ambrose A, Boivin G, et al. Influenza vaccine effectiveness to prevent influenza-related hospitalizations and serious outcomes in Canadian adults over the 2011/12 through 2013/14 influenza seasons: A pooled analysis from the Canadian Immunization Research Network (CIRN) Serious Outcomes Surveillance (SOS Network) Vaccine. 2018;36:2166–2175. doi: 10.1016/j.vaccine.2018.02.093. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

