Adipocyte and lipid metabolism in cancer drug resistance
- PMID: 31264969
- PMCID: PMC6668696
- DOI: 10.1172/JCI127201
Adipocyte and lipid metabolism in cancer drug resistance
Abstract
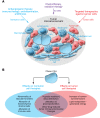
Development of novel and effective therapeutics for treating various cancers is probably the most congested and challenging enterprise of pharmaceutical companies. Diverse drugs targeting malignant and nonmalignant cells receive clinical approval each year from the FDA. Targeting cancer cells and nonmalignant cells unavoidably changes the tumor microenvironment, and cellular and molecular components relentlessly alter in response to drugs. Cancer cells often reprogram their metabolic pathways to adapt to environmental challenges and facilitate survival, proliferation, and metastasis. While cancer cells' dependence on glycolysis for energy production is well studied, the roles of adipocytes and lipid metabolic reprogramming in supporting cancer growth, metastasis, and drug responses are less understood. This Review focuses on emerging mechanisms involving adipocytes and lipid metabolism in altering the response to cancer treatment. In particular, we discuss mechanisms underlying cancer-associated adipocytes and lipid metabolic reprogramming in cancer drug resistance.
Conflict of interest statement
Figures


References
-
- Liu Y, Zuckier LS, Ghesani NV. Dominant uptake of fatty acid over glucose by prostate cells: a potential new diagnostic and therapeutic approach. Anticancer Res. 2010;30(2):369–374. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

