Sex Difference in Corticosterone-Induced Insulin Resistance in Mice
- PMID: 31265057
- PMCID: PMC6760317
- DOI: 10.1210/en.2019-00194
Sex Difference in Corticosterone-Induced Insulin Resistance in Mice
Abstract
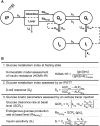
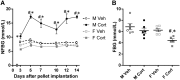
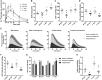
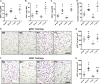
Prolonged exposure to glucocorticoids (GCs) causes various metabolic derangements. These include obesity and insulin resistance, as inhibiting glucose utilization in adipose tissues is a major function of GCs. Although adipose tissue distribution and glucose homeostasis are sex-dependently regulated, it has not been evaluated whether GCs affect glucose metabolism and adipose tissue functions in a sex-dependent manner. In this study, high-dose corticosterone (rodent GC) treatment in C57BL/6J mice resulted in nonfasting hyperglycemia in male mice only, whereas both sexes displayed hyperinsulinemia with normal fasting glucose levels, indicative of insulin resistance. Metabolic testing using stable isotope-labeled glucose techniques revealed a sex-specific corticosterone-driven glucose intolerance. Corticosterone treatment increased adipose tissue mass in both sexes, which was reflected by elevated serum leptin levels. However, female mice showed more metabolically protective adaptations of adipose tissues than did male mice, demonstrated by higher serum total and high-molecular-weight adiponectin levels, more hyperplastic morphological changes, and a stronger increase in mRNA expression of adipogenic differentiation markers. Subsequently, in vitro studies in 3T3-L1 (white) and T37i (brown) adipocytes suggest that the increased leptin and adiponectin levels were mainly driven by the elevated insulin levels. In summary, this study demonstrates that GC-induced insulin resistance is more severe in male mice than in female mice, which can be partially explained by a sex-dependent adaptation of adipose tissues.
Copyright © 2019 Endocrine Society.
Conflict of interest statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Figures





Similar articles
-
Androgens sensitise mice to glucocorticoid-induced insulin resistance and fat accumulation.Diabetologia. 2019 Aug;62(8):1463-1477. doi: 10.1007/s00125-019-4887-0. Epub 2019 May 16. Diabetologia. 2019. PMID: 31098671
-
Glucocorticoid-induced insulin resistance is related to macrophage visceral adipose tissue infiltration.J Steroid Biochem Mol Biol. 2019 Jan;185:150-162. doi: 10.1016/j.jsbmb.2018.08.010. Epub 2018 Aug 23. J Steroid Biochem Mol Biol. 2019. PMID: 30145227
-
Activation of the hexosamine signaling pathway in adipose tissue results in decreased serum adiponectin and skeletal muscle insulin resistance.Endocrinology. 2004 May;145(5):2118-28. doi: 10.1210/en.2003-0812. Epub 2003 Dec 18. Endocrinology. 2004. PMID: 14684615
-
The role of sex in the relationship between fasting adipokines levels, maximal fat oxidation during exercise, and insulin resistance in young adults with excess adiposity.Biochem Pharmacol. 2023 Oct;216:115757. doi: 10.1016/j.bcp.2023.115757. Epub 2023 Aug 19. Biochem Pharmacol. 2023. PMID: 37598975
-
High-fat diet and glucocorticoid treatment cause hyperglycemia associated with adiponectin receptor alterations.Lipids Health Dis. 2011 Jan 18;10:11. doi: 10.1186/1476-511X-10-11. Lipids Health Dis. 2011. PMID: 21244702 Free PMC article.
Cited by
-
Sexual Dimorphism in Adipose-Hypothalamic Crosstalk and the Contribution of Aryl Hydrocarbon Receptor to Regulate Energy Homeostasis.Int J Mol Sci. 2022 Jul 12;23(14):7679. doi: 10.3390/ijms23147679. Int J Mol Sci. 2022. PMID: 35887027 Free PMC article. Review.
-
Sex-based impact of pancreatic islet stressors in GluCreERT2/Rosa26-eYFP mice.J Endocrinol. 2023 Sep 25;259(2):e230174. doi: 10.1530/JOE-23-0174. Print 2023 Nov 1. J Endocrinol. 2023. PMID: 37650517 Free PMC article.
-
Dexamethasone-Induced Insulin Resistance Attenuation by Oral Sulfur-Oxidovanadium(IV) Complex Treatment in Mice.Pharmaceuticals (Basel). 2024 Jun 10;17(6):760. doi: 10.3390/ph17060760. Pharmaceuticals (Basel). 2024. PMID: 38931427 Free PMC article.
-
Glucocorticoids, stress and eating: The mediating role of appetite-regulating hormones.Obes Rev. 2023 Mar;24(3):e13539. doi: 10.1111/obr.13539. Epub 2022 Dec 8. Obes Rev. 2023. PMID: 36480471 Free PMC article. Review.
-
Sexual Dimorphism in Transcriptional and Functional Glucocorticoid Effects on Mouse Skeletal Muscle.Front Endocrinol (Lausanne). 2022 Jul 11;13:907908. doi: 10.3389/fendo.2022.907908. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35898460 Free PMC article.
References
-
- Juszczak A, Morris DG, Grossman AB, Nieman LK. Cushing’s syndrome. In: Jameson JL, De Groot LJ, de Kretser DM, Giudice LC, Grossman AB, Melmed S, Potts JT Jr, Weir GC, eds. Endocrinology: Adult and Pediatric. 7th ed Philadelphia, PA: Elsevier Saunders; 2016:227–255.e11.
-
- Magomedova L, Cummins CL. Glucocorticoids and metabolic control. Handb Exp Pharmacol. 2016;233:73–93. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

