Comparison of Teriparatide and Denosumab in Patients Switching From Long-Term Bisphosphonate Use
- PMID: 31265071
- PMCID: PMC6785688
- DOI: 10.1210/jc.2019-00924
Comparison of Teriparatide and Denosumab in Patients Switching From Long-Term Bisphosphonate Use
Abstract
Context: Teriparatide and denosumab are effective treatments for osteoporosis and typically reserved as second-line options after patients have used bisphosphonates. However, limited head-to-head comparative effectiveness data exist between teriparatide and denosumab.
Objective: We compared changes in bone mineral density (BMD) between groups treated with teriparatide or denosumab after using bisphosphonates, focusing on the change in BMD while on either drug over 2 years.
Design: Observational cohort study using electronic medical records from two academic medical centers in the United States.
Participants: The study population included osteoporotic patients >45 years who received bisphosphonates >1 year before switching to teriparatide or denosumab.
Outcome measures: Annualized BMD change from baseline at the lumbar spine, total hip, and femoral neck.
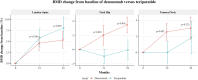
Results: Patients treated with teriparatide (n = 110) were compared with those treated with denosumab (n = 105); the mean (SD) age was 70 (10) years and median duration (interquartile range) of bisphosphonate use was 7.0 (5.6 to 9.7) years. Compared with denosumab users, teriparatide users had higher annualized BMD change at the spine by 1.3% (95% CI 0.02, 2.7%) but lower at the total hip by -2.2% (95% CI -2.9 to -1.5%) and the femoral neck by -1.1% (95% CI -2.1 to -0.1%). Those who switched to teriparatide had a transient loss of hip BMD for the first year, with no overall increase in the total hip BMD over 2 years.
Conclusions: Among patients who use long-term bisphosphonates, the decision of switching to teriparatide should be made with caution, especially for patients at high risk of hip fracture.
Copyright © 2019 Endocrine Society.
Figures


Comment in
-
Letter to the Editor: "Comparison of Teriparatide and Denosumab in Patients Switching from Long-Term Bisphosphonate Use".J Clin Endocrinol Metab. 2019 Dec 1;104(12):5913-5914. doi: 10.1210/jc.2019-01650. J Clin Endocrinol Metab. 2019. PMID: 31408164 No abstract available.
-
Letter to the Editor: "Comparison of Teriparatide and Denosumab in Patients Switching from Long-Term Bisphosphonate Use".J Clin Endocrinol Metab. 2020 Mar 1;105(3):dgz122. doi: 10.1210/clinem/dgz122. J Clin Endocrinol Metab. 2020. PMID: 31650166 No abstract available.
-
Reply to Letter to the Editor: "Comparison of Teriparatide and Denosumab in Patients Switching from Long-Term Bisphosphonate Use".J Clin Endocrinol Metab. 2020 May 1;105(5):dgz123. doi: 10.1210/clinem/dgz123. J Clin Endocrinol Metab. 2020. PMID: 31650171 No abstract available.
Similar articles
-
Bisphosphonates Maintain BMD After Sequential Teriparatide and Denosumab in Premenopausal Women with Idiopathic Osteoporosis.J Clin Endocrinol Metab. 2025 Feb 18;110(3):e791-e801. doi: 10.1210/clinem/dgae240. J Clin Endocrinol Metab. 2025. PMID: 38605469 Free PMC article.
-
Four-Year Teriparatide Followed by Denosumab vs. Continuous Denosumab in Glucocorticoid-Induced Osteoporosis Patients With Prior Bisphosphonate Treatment.Front Endocrinol (Lausanne). 2021 Sep 27;12:753185. doi: 10.3389/fendo.2021.753185. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34646240 Free PMC article. Clinical Trial.
-
Assessment of the effects of switching oral bisphosphonates to denosumab or daily teriparatide in patients with rheumatoid arthritis.J Bone Miner Metab. 2018 Jul;36(4):478-487. doi: 10.1007/s00774-017-0861-4. Epub 2017 Aug 1. J Bone Miner Metab. 2018. PMID: 28766140
-
Efficacy and safety of teriparatide vs. bisphosphonates and denosumab vs. bisphosphonates in osteoporosis not previously treated with bisphosphonates: a systematic review and meta-analysis of randomized controlled trials.Arch Osteoporos. 2024 Sep 23;19(1):89. doi: 10.1007/s11657-024-01447-7. Arch Osteoporos. 2024. PMID: 39312040 Free PMC article.
-
Denosumab, raloxifene, romosozumab and teriparatide to prevent osteoporotic fragility fractures: a systematic review and economic evaluation.Health Technol Assess. 2020 Jun;24(29):1-314. doi: 10.3310/hta24290. Health Technol Assess. 2020. PMID: 32588816 Free PMC article.
Cited by
-
Osteoporosis in 10 years time: a glimpse into the future of osteoporosis.Ther Adv Musculoskelet Dis. 2022 Mar 20;14:1759720X221083541. doi: 10.1177/1759720X221083541. eCollection 2022. Ther Adv Musculoskelet Dis. 2022. PMID: 35342458 Free PMC article.
-
Skeletal outcomes of patients with osteogenesis imperfecta during drug holiday of bisphosphonates: a real-world study.Front Endocrinol (Lausanne). 2022 Sep 26;13:901925. doi: 10.3389/fendo.2022.901925. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36225201 Free PMC article.
-
Asia-Pacific consensus on long-term and sequential therapy for osteoporosis.Osteoporos Sarcopenia. 2024 Mar;10(1):3-10. doi: 10.1016/j.afos.2024.02.001. Epub 2024 Mar 16. Osteoporos Sarcopenia. 2024. PMID: 38690538 Free PMC article. Review.
-
MTX Osteopathy Versus Osteoporosis Including Response to Treatment Data-A Retrospective Single Center Study Including 172 Patients.Calcif Tissue Int. 2024 Nov;115(5):599-610. doi: 10.1007/s00223-024-01290-5. Epub 2024 Sep 25. Calcif Tissue Int. 2024. PMID: 39322780 Free PMC article.
-
Denosumab in the Treatment of Osteoporosis: 10 Years Later: A Narrative Review.Adv Ther. 2022 Jan;39(1):58-74. doi: 10.1007/s12325-021-01936-y. Epub 2021 Nov 11. Adv Ther. 2022. PMID: 34762286 Free PMC article. Review.
References
-
- Parikh S, Mogun H, Avorn J, Solomon DH. Osteoporosis medication use in nursing home patients with fractures in 1 US state. Arch Intern Med. 2008;168(10):1111–1115. - PubMed
-
- Yusuf AA, Matlon TJ, Grauer A, Barron R, Chandler D, Peng Y. Utilization of osteoporosis medication after a fragility fracture among elderly Medicare beneficiaries. Arch Osteoporos. 2016;11(1):31. - PubMed
-
- Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis [published correction appears in N Engl J Med. 2009;361(19):1914]. N Engl J Med. 2009;361(8):756–765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

