Targeted Gold Nanocluster-Enhanced Radiotherapy of Prostate Cancer
- PMID: 31265213
- PMCID: PMC6707872
- DOI: 10.1002/smll.201900968
Targeted Gold Nanocluster-Enhanced Radiotherapy of Prostate Cancer
Abstract
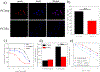
For over a hundred years, X-rays have been a main component of the radiotherapeutic approaches to treat cancer. Yet, to date, no radiosensitizer has been developed to selectively target prostate cancer. Gold has excellent X-ray absorptivity and is used as a radiotherapy enhancing material. In this work, ultrasmall Au25 nanoclusters (NCs) are developed for selective prostate cancer targeting, radiotherapy enhancement, and rapid clearance from the body. Targeted-Au25 NCs are rapidly and selectively taken up by prostate cancer in vitro and in vivo and also have fast renal clearance. When combined with X-ray irradiation of the targeted cancer tissues, radiotherapy is significantly enhanced. The selective targeting and rapid clearance of the nanoclusters may allow reductions in radiation dose, decreasing exposure to healthy tissue and making them highly attractive for clinical translation.
Keywords: PSMA; gold nanoclusters; prostate cancer; radiosensitization; targeted radiotherapy.
© 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest.
Figures





References
-
- Siegel RL, Miller KD, Jemal A, CA Cancer J. Clin 2018, 68, 7. - PubMed
-
- Wang X, Tsui B, Ramamurthy G, Zhang P, Meyers J, Kenney ME, Kiechle J, Ponsky L, Basilion JP, Mol. Cancer Ther 2016, 15, 1834. - PubMed
-
- Zhu S, Gu Z, Zhao Y, Adv. Ther 2018, 1, 1800050.
-
- Song G, Cheng L, Chao Y, Yang K, Liu Z, Adv. Mater 2017, 29, 1700996. - PubMed
-
- Hainfeld JF, Dilmanian FA, Slatkin DN, Smilowitz HM, J. Pharm. Pharmacol 2008, 60, 977. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

