First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia
- PMID: 31266741
- PMCID: PMC6616260
- DOI: 10.1182/bloodadvances.2018028332
First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia
Abstract
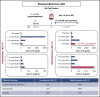
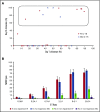
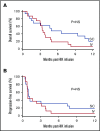
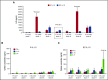
In vivo expansion of haploidentical natural killer (NK) cell infusions with interleukin-2 (IL-2) can induce remission of refractory acute myeloid leukemia, but efficacy may be hampered by concurrent stimulation of host regulatory T cells. To overcome this limitation, we substituted the NK homeostatic factor IL-15 in 2 phase 1/2 trials. Forty-two patients received either intravenous (IV) (NCT01385423) or subcutaneous (SC) (NCT02395822) recombinant human IL-15 (rhIL-15) after lymphodepleting chemotherapy and haploidentical NK cells. Escalating doses of rhIL-15 (0.3-1.0 μg/kg) were given on 12 consecutive days in a phase 1 trial. Of 26 patients, 36% had robust in vivo NK-cell expansion at day 14, and 32% achieved complete remission. Hypothesizing that SC dosing of rhIL-15 would be safer and better tolerated, 16 patients received 10 once per day doses of SC rhIL-15 at 2.0 μg/kg on a phase 2 trial. NK-cell expansion at day 14 was seen in 27% of the patients, and 40% achieved remission. rhIL-15 induced better rates of in vivo NK-cell expansion and remission compared with previous trials with IL-2, but it was associated with previously unreported cytokine release syndrome (CRS) after SC but not IV dosing. CRS was observed in 56% of patients given SC rhIL-15 (with concurrent neurologic toxicity in 5 of 9 patients) and was responsive to steroids and tocilizumab. SC administration was associated with slower pharmacokinetic clearance and higher levels of IL-6 than IV dosing. These novel trials testing the use of IL-15 to potentiate cell therapy suggest that dosing schedules based on pharmacokinetics and pharmacodynamics will preserve the therapeutic benefits of IL-15 and minimize CRS. These trials were registered at www.clinicaltrials.gov as #NCT01385423 and #NCT02395822.
Conflict of interest statement
Conflict-of-interest disclosure: S.C., who contributed to this work while on faculty at University of Minnesota, is now an employee of Fate Therapeutics. The remaining authors declare no competing financial interests.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

