Two complementary α-fucosidases from Streptococcus pneumoniae promote complete degradation of host-derived carbohydrate antigens
- PMID: 31266803
- PMCID: PMC6709623
- DOI: 10.1074/jbc.RA119.009368
Two complementary α-fucosidases from Streptococcus pneumoniae promote complete degradation of host-derived carbohydrate antigens
Abstract
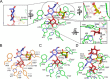
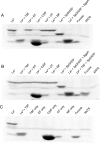
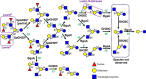
An important aspect of the interaction between the opportunistic bacterial pathogen Streptococcus pneumoniae and its human host is its ability to harvest host glycans. The pneumococcus can degrade a variety of complex glycans, including N- and O-linked glycans, glycosaminoglycans, and carbohydrate antigens, an ability that is tightly linked to the virulence of S. pneumoniae Although S. pneumoniae is known to use a sophisticated enzyme machinery to attack the human glycome, how it copes with fucosylated glycans, which are primarily histo-blood group antigens, is largely unknown. Here, we identified two pneumococcal enzymes, SpGH29C and SpGH95C, that target α-(1→3/4) and α-(1→2) fucosidic linkages, respectively. X-ray crystallography studies combined with functional assays revealed that SpGH29C is specific for the LewisA and LewisX antigen motifs and that SpGH95C is specific for the H(O)-antigen motif. Together, these enzymes could defucosylate LewisY and LewisB antigens in a complementary fashion. In vitro reconstruction of glycan degradation cascades disclosed that the individual or combined activities of these enzymes expose the underlying glycan structure, promoting the complete deconstruction of a glycan that would otherwise be resistant to pneumococcal enzymes. These experiments expand our understanding of the extensive capacity of S. pneumoniae to process host glycans and the likely roles of α-fucosidases in this. Overall, given the importance of enzymes that initiate glycan breakdown in pneumococcal virulence, such as the neuraminidase NanA and the mannosidase SpGH92, we anticipate that the α-fucosidases identified here will be important factors in developing more refined models of the S. pneumoniae-host interaction.
Keywords: Streptococcus; X-ray crystallography; glycoside hydrolase; host-pathogen interaction; structure-function.
© 2019 Hobbs et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
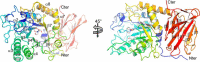


References
-
- Varki A., and Marth J. (1995) Oligosaccharides in vertebrate development. Semin. Dev. Biol. 6, 127–138 10.1016/S1044-5781(06)80022-8 - DOI
-
- Gagneux P., Aebi M., and Varki A. (2015) Evolution of glycan diversity, in Essentials of Glycobiology (Varki A., Cummings R. D., Esko J. D., Stanley P., Hart G. W., Aebi M., Darvill A. G., Kinoshita T., Packer N. H., Prestegard J. H., Schnaar R. L., and Seeberger P. H., eds) 3rd Ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, NY - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

