An Intermolecular π-Stacking Interaction Drives Conformational Changes Necessary to β-Barrel Formation in a Pore-Forming Toxin
- PMID: 31266869
- PMCID: PMC6606804
- DOI: 10.1128/mBio.01017-19
An Intermolecular π-Stacking Interaction Drives Conformational Changes Necessary to β-Barrel Formation in a Pore-Forming Toxin
Abstract
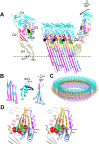
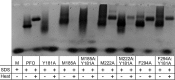
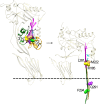
The crystal structures of the soluble monomers of the pore-forming cholesterol-dependent cytolysins (CDCs) contain two α-helical bundles that flank a twisted core β-sheet. This protein fold is the hallmark of the CDCs, as well as of the membrane attack complex/perforin immune defense proteins and the stonefish toxins. To form the β-barrel pore, a core β-sheet is flattened to align the membrane-spanning β-hairpins. Concomitantly with this conformational change, the two α-helical bundles that flank the core β-sheet break their restraining contacts and refold into two membrane-spanning β-hairpins of the β-barrel pore. The studies herein show that in the monomer structure of the archetype CDC perfringolysin O (PFO), a conserved Met-Met-Phe triad simultaneously contributes to maintaining the twist in this core β-sheet, as well as restricting the α-helical-to-β-strand transition necessary to form one of two membrane-spanning β-hairpins. A previously identified intermolecular π-stacking interaction is now shown to disrupt the interactions mediated by this conserved triad. This is required to establish the subsequent intermolecular electrostatic interaction, which has previously been shown to drive the final conformational changes necessary to form the β-barrel pore. Hence, these studies show that the intermolecular π-stacking and electrostatic interactions work in tandem to flatten the core β-sheet and initiate the α-helical-to-β-strand transitions to form the β-barrel pore.IMPORTANCE A unique feature of the CDC/MACPF/SNTX (cholesterol-dependent cytolysin/membrane attack complex perforin/stonefish toxin) superfamily of pore-forming toxins is that the β-strands that comprise the β-barrel pore are derived from a pair of α-helical bundles. These studies reveal the molecular basis by which the formation of intermolecular interactions within the prepore complex drive the disruption of intramolecular interactions within each monomer of the prepore to trigger the α-helical-to-β-strand transition and formation of the β-barrel pore.
Keywords: cholesterol-dependent cytolysin; membrane attack complex; oligomer; perforin; β-barrel.
Copyright © 2019 Burns et al.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
