Transient drug-tolerance and permanent drug-resistance rely on the trehalose-catalytic shift in Mycobacterium tuberculosis
- PMID: 31266959
- PMCID: PMC6606615
- DOI: 10.1038/s41467-019-10975-7
Transient drug-tolerance and permanent drug-resistance rely on the trehalose-catalytic shift in Mycobacterium tuberculosis
Abstract
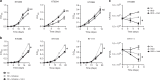
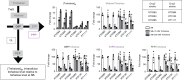
Stochastic formation of Mycobacterium tuberculosis (Mtb) persisters achieves a high level of antibiotic-tolerance and serves as a source of multidrug-resistant (MDR) mutations. As conventional treatment is not effective against infections by persisters and MDR-Mtb, novel therapeutics are needed. Several approaches were proposed to kill persisters by altering their metabolism, obviating the need to target active processes. Here, we adapted a biofilm culture to model Mtb persister-like bacilli (PLB) and demonstrated that PLB underwent trehalose metabolism remodeling. PLB use trehalose as an internal carbon to biosynthesize central carbon metabolism intermediates instead of cell surface glycolipids, thus maintaining levels of ATP and antioxidants. Similar changes were identified in Mtb following antibiotic-treatment, and MDR-Mtb as mechanisms to circumvent antibiotic effects. This suggests that trehalose metabolism is associated not only with transient drug-tolerance but also permanent drug-resistance, and serves as a source of adjunctive therapeutic options, potentiating antibiotic efficacy by interfering with adaptive strategies.
Conflict of interest statement
The authors declare no competing interests.
Figures



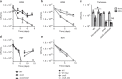

References
-
- WHO. Global tuberculosis report (2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

