"Stuck on sugars - how carbohydrates regulate cell adhesion, recognition, and signaling"
- PMID: 31267247
- PMCID: PMC6657361
- DOI: 10.1007/s10719-019-09876-0
"Stuck on sugars - how carbohydrates regulate cell adhesion, recognition, and signaling"
Abstract
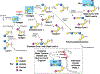
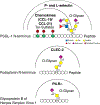
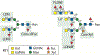
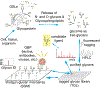
We have explored the fundamental biological processes by which complex carbohydrates expressed on cellular glycoproteins and glycolipids and in secretions of cells promote cell adhesion and signaling. We have also explored processes by which animal pathogens, such as viruses, bacteria, and parasites adhere to glycans of animal cells and initiate disease. Glycans important in cell signaling and adhesion, such as key O-glycans, are essential for proper animal development and cellular differentiation, but they are also involved in many pathogenic processes, including inflammation, tumorigenesis and metastasis, and microbial and parasitic pathogenesis. The overall hypothesis guiding these studies is that glycoconjugates are recognized and bound by a growing class of proteins called glycan-binding proteins (GBPs or lectins) expressed by all types of cells. There is an incredible variety and diversity of GBPs in animal cells involved in binding N- and O-glycans, glycosphingolipids, and proteoglycan/glycosaminoglycans. We have specifically studied such molecular determinants recognized by selectins, galectins, and many other C-type lectins, involved in leukocyte recruitment to sites of inflammation in human tissues, lymphocyte trafficking, adhesion of human viruses to human cells, structure and immunogenicity of glycoproteins on the surfaces of human parasites. We have also explored the molecular basis of glycoconjugate biosynthesis by exploring the enzymes and molecular chaperones required for correct protein glycosylation. From these studies opportunities for translational biology have arisen, involving production of function-blocking antibodies, anti-glycan specific antibodies, and synthetic glycoconjugates, e.g. glycosulfopeptides, that specifically are recognized by GBPs. This invited short review is based in part on my presentation for the IGO Award 2019 given by the International Glycoconjugate Organization in Milan.
Keywords: Antibodies; Cosmc; Glycobiology; Glycomics; Glycoproteins; Immunity; Inflammation; Parasites; Selectins; T-synthase.
Figures

References
-
- Cummings RD, Schnaar RL, Esko JD, Drickamer K, Taylor ME: Principles of Glycan Recognition In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH (eds.) Essentials of Glycobiology. pp. 373–385. Cold Spring Harbor; (NY: ) (2017)
-
- Kaltner H, Toegel S, Caballero GG, Manning JC, Ledeen RW, Gabius HJ: Galectins: their network and roles in immunity/tumor growth control. Histochem Cell Biol 147(2), 239–256 (2017) - PubMed
-
- Johannes L, Jacob R, Leffler H: Galectins at a glance. J Cell Sci 131(9) (2018). - PubMed
-
- Mendez-Huergo SP, Blidner AG, Rabinovich GA: Galectins: emerging regulatory checkpoints linking tumor immunity and angiogenesis. Curr Opin Immunol 45, 8–15 (2017) - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

