Serological Profiling for Malaria Surveillance Using a Standard ELISA Protocol
- PMID: 31267495
- PMCID: PMC7613324
- DOI: 10.1007/978-1-4939-9550-9_6
Serological Profiling for Malaria Surveillance Using a Standard ELISA Protocol
Abstract
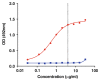
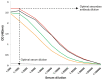
The enzyme-linked immunosorbent assay (ELISA) is a reliable and relatively low-cost method for measuring soluble ligands such as antibodies and proteins in biological samples. For analysis of specific antibodies in serum, a capture antigen is immobilized onto a solid polystyrene surface from which it can capture the antibodies. The captured antibodies are subsequently detected using a secondary antibody conjugated to an enzyme. Detection is accomplished by addition of a colorimetric substrate, and the readout is absorbance (optical density). Here, we provide a detailed standardized ELISA protocol for the quantification of antibodies against malaria antigens.
Keywords: Antibodies; Antigens; ELISA; Optical density; Serum.
Figures
References
-
- Vignali DA. Multiplexed particle-based flow cytometric assays. J Immunol Methods. 2000;243:243–255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

