Impact of the acute local inhibition of soluble epoxide hydrolase on diabetic skin microcirculatory dysfunction
- PMID: 31267765
- PMCID: PMC7307659
- DOI: 10.1177/1479164119860215
Impact of the acute local inhibition of soluble epoxide hydrolase on diabetic skin microcirculatory dysfunction
Abstract
The impact of the local inhibition of soluble epoxide hydrolase, which metabolizes vasodilator and anti-inflammatory epoxyeicosanoids, on diabetic skin microvascular dysfunction was assessed. In diabetic db/db mice, basal skin blood flow assessed using laser Doppler imaging was similar to that of control mice, but thermal hyperemia was markedly reduced. At 2 h after the topical administration of an aqueous gel containing the soluble epoxide hydrolase inhibitor trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid (t-AUCB: 400 mg/L), the peak concentration of t-AUCB was detected in the skin of diabetic mice, which quickly decreased thereafter. In parallel, 2 h after application of t-AUCB treatment, thermal hyperemia was increased compared to the control gel. Quantification of t-AUCB in plasma of treated animals showed no or low systemic diffusion. Furthermore, haematoxylin and eosin histological staining of skin biopsies showed that skin integrity was preserved in t-AUCB-treated mice. Finally, for pig ear skin, a surrogate for human skin, using Franz diffusion cells, we observed a continuous diffusion of t-AUCB from 2 h after application to beyond 24 h. A single topical administration of a soluble epoxide hydrolase inhibitor improves microcirculatory function in the skin of db/db mice and might represent a new therapeutic approach for preventing the development of skin complications in diabetic patients.
Keywords: Diabetes; skin microvascular dysfunction; soluble epoxide hydrolase; topical form.
Conflict of interest statement
DECLARATION OF CONFLICTING INTERESTS
The author(s) declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Figures

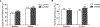
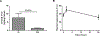
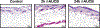

Similar articles
-
Pharmacokinetic optimization of four soluble epoxide hydrolase inhibitors for use in a murine model of inflammation.Br J Pharmacol. 2009 Jan;156(2):284-96. doi: 10.1111/j.1476-5381.2008.00009.x. Epub 2009 Jan 13. Br J Pharmacol. 2009. PMID: 19154430 Free PMC article.
-
Soluble epoxide hydrolase inhibition improves coronary endothelial function and prevents the development of cardiac alterations in obese insulin-resistant mice.Am J Physiol Heart Circ Physiol. 2015 May 1;308(9):H1020-9. doi: 10.1152/ajpheart.00465.2014. Epub 2015 Feb 27. Am J Physiol Heart Circ Physiol. 2015. PMID: 25724490 Free PMC article.
-
Role of haem oxygenase in the renoprotective effects of soluble epoxide hydrolase inhibition in diabetic spontaneously hypertensive rats.Clin Sci (Lond). 2013 Oct;125(7):349-59. doi: 10.1042/CS20130003. Clin Sci (Lond). 2013. PMID: 23611540
-
Meloxicam fails to augment the reno-protective effects of soluble epoxide hydrolase inhibition in streptozotocin-induced diabetic rats via increased 20-HETE levels.Prostaglandins Other Lipid Mediat. 2017 Sep;132:3-11. doi: 10.1016/j.prostaglandins.2016.08.004. Epub 2016 Sep 3. Prostaglandins Other Lipid Mediat. 2017. PMID: 27596333 Review.
-
Diabetes and microvascular pathophysiology: role of epidermal growth factor receptor tyrosine kinase.Diabetes Metab Res Rev. 2010 Jan;26(1):13-6. doi: 10.1002/dmrr.1050. Diabetes Metab Res Rev. 2010. PMID: 19943320 Free PMC article. Review.
Cited by
-
Role of the soluble epoxide hydrolase in keratinocyte proliferation and sensitivity of skin to inflammatory stimuli.Biomed Pharmacother. 2024 Feb;171:116127. doi: 10.1016/j.biopha.2024.116127. Epub 2024 Jan 9. Biomed Pharmacother. 2024. PMID: 38198951 Free PMC article.
-
Small Molecule Soluble Epoxide Hydrolase Inhibitors in Multitarget and Combination Therapies for Inflammation and Cancer.Molecules. 2020 Nov 24;25(23):5488. doi: 10.3390/molecules25235488. Molecules. 2020. PMID: 33255197 Free PMC article. Review.
References
-
- Moxey PW, Hofman D, Hinchliffe RJ, Jones K, Thompson MM, Holt PJE. Epidemiological study of lower limb amputation in England between 2003 and 2008. Br J Surg. 2010;97:1348–1353. - PubMed
-
- Chao CY, Cheing GL. Microvascular dysfunction in diabetic foot disease and ulceration. Diabetes Metab Res Rev. 2009;25:604–614. - PubMed
-
- Jhamb S, Vangaveti VN, Malabu UH. Genetic and molecular basis of diabetic foot ulcers: Clinical review. J Tissue Viability. 2016;25:229–236. - PubMed
-
- Valacchi G, Zanardi I, Sticozzi C, Bocci V, Travagli V. Emerging topics in cutaneous wound repair. Ann N Y Acad Sci. 2012;1259:136–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

