A Modeling Framework to Characterize Cytokine Release upon T-Cell-Engaging Bispecific Antibody Treatment: Methodology and Opportunities
- PMID: 31268236
- PMCID: PMC6853151
- DOI: 10.1111/cts.12662
A Modeling Framework to Characterize Cytokine Release upon T-Cell-Engaging Bispecific Antibody Treatment: Methodology and Opportunities
Abstract
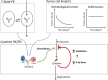
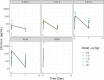
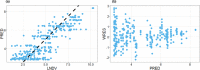
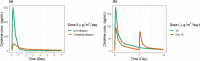
T-cell-engaging bispecific antibodies (T-BsAbs) are an important class of antibody therapeutics in immuno-oncology. T-BsAbs simultaneously bind to CD3 on T cells and a tumor-associated antigen on tumor cells, activate T cells, and redirect T cells' cytotoxicity against tumor cells. Cytokine release syndrome (CRS), a common dose-limiting adverse event for T-BsAbs, is associated with T-cell activation. A "priming" dose strategy (i.e., a lower initial dose followed by a higher maintenance dose) has been implemented in the clinic to mitigate CRS and to achieve efficacious doses with T-BsAbs. So far, the selection of the optimal priming dosing regimen is largely empirical. A "fit-for-purpose" semimechanistic pharmacokinetic/pharmacodynamic model was developed to characterize the cytokine release profiles upon T-BsAb treatment, including the priming effect observed with repeated dosing. This model can be utilized to simulate cytokine profiles following various dosing regimens and may assist the design of clinical dosing strategies for T-BsAbs programs.
© 2019 Pfizer Inc. Clinical and Translational Science published by Wiley Periodicals Inc. on behalf of the American Society of Clinical Pharmacology & Therapeutics.
Conflict of interest statement
X.C., C.K., G.W., and D.X. are employed by Pfizer and hold stock in Pfizer.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

