Cost-Effectiveness of Alternative Uses of Polyvalent Meningococcal Vaccines in Niger: An Agent-Based Transmission Modeling Study
- PMID: 31268405
- PMCID: PMC6786941
- DOI: 10.1177/0272989X19859899
Cost-Effectiveness of Alternative Uses of Polyvalent Meningococcal Vaccines in Niger: An Agent-Based Transmission Modeling Study
Abstract
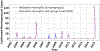
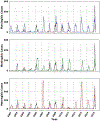
Background. Despite the introduction of an effective serogroup A conjugate vaccine (MenAfriVac™), sporadic epidemics of other Neisseria meningitidis serogroups remain a concern in Africa. Polyvalent meningococcal conjugate (PMC) vaccines may offer alternatives to current strategies that rely on routine infant vaccination with MenAfriVac plus, in the event of an epidemic, district-specific reactive campaigns using polyvalent meningococcal polysaccharide (PMP) vaccines. Methods. We developed an agent-based transmission model of N. meningitidis in Niger to compare the health effects and costs of current vaccination practice and 3 alternatives. Each alternative replaces MenAfriVac in the infant vaccination series with PMC and either replaces PMP with PMC for reactive campaigns or implements a one-time catch up campaign with PMC for children and young adults. Results. Over a 28-year period, replacement of MenAfriVac with PMC in the infant immunization series and of PMP in reactive campaigns would avert 63% of expected cases (95% prediction interval 49%-75%) if elimination of serogroup A is not followed by serogroup replacement. At a PMC price of $4/dose, this would cost $1412 ($81-$3510) per disability-adjusted life-year (DALY) averted. If serogroup replacement occurs, the cost-effectiveness of this strategy improves to $662 (cost-saving, $2473) per DALY averted. Sensitivity analyses accounting for incomplete laboratory confirmation suggest that a catch-up PMC campaign would also meet standard cost-effectiveness thresholds. Limitations. The assumption that polyvalent vaccines offer similar protection against all serogroups is simplifying. Conclusions. The use of PMC vaccines to replace MenAfriVac in routine infant immunization and in district-specific reactive campaigns would have important health benefits and is likely to be cost-effective in Niger. An additional PMC catch-up campaign would also be cost-effective if we account for incomplete laboratory reporting.
Keywords: economic evaluation; meningitis; meningococcal; simulation; vaccine.
Figures


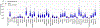




Similar articles
-
The cost-effectiveness of alternative vaccination strategies for polyvalent meningococcal vaccines in Burkina Faso: A transmission dynamic modeling study.PLoS Med. 2018 Jan 24;15(1):e1002495. doi: 10.1371/journal.pmed.1002495. eCollection 2018 Jan. PLoS Med. 2018. PMID: 29364884 Free PMC article.
-
Successful African introduction of a new Group A meningococcal conjugate vaccine: Future challenges and next steps.Hum Vaccin Immunother. 2018 May 4;14(5):1098-1102. doi: 10.1080/21645515.2017.1378841. Epub 2017 Nov 8. Hum Vaccin Immunother. 2018. PMID: 28968148 Free PMC article.
-
Economics of an adolescent meningococcal conjugate vaccination catch-up campaign in the United States.Clin Infect Dis. 2008 Jan 1;46(1):1-13. doi: 10.1086/524041. Clin Infect Dis. 2008. PMID: 18171206
-
An evaluation of emerging vaccines for childhood meningococcal disease.BMC Public Health. 2011 Apr 13;11 Suppl 3(Suppl 3):S29. doi: 10.1186/1471-2458-11-S3-S29. BMC Public Health. 2011. PMID: 21501447 Free PMC article. Review.
-
Neisseria meningitidis serogroup A vaccines: an overview.Expert Rev Vaccines. 2003 Aug;2(4):571-82. doi: 10.1586/14760584.2.4.571. Expert Rev Vaccines. 2003. PMID: 14711341 Review.
Cited by
-
Public health impact and cost-effectiveness of introducing MenACWY vaccination strategies in Germany.BMC Public Health. 2025 May 5;25(1):1653. doi: 10.1186/s12889-025-21491-3. BMC Public Health. 2025. PMID: 40325417 Free PMC article.
-
Vaccines to Prevent Meningitis: Historical Perspectives and Future Directions.Microorganisms. 2021 Apr 7;9(4):771. doi: 10.3390/microorganisms9040771. Microorganisms. 2021. PMID: 33917003 Free PMC article. Review.
References
-
- World Health Organization, “Meningitis Outbreak Response in Sub-Saharan Africa: WHO Guideline, Report No. WHO/HSE/PED/CED/14.5,” 2014. - PubMed
-
- McIntyre PB, O’Brien KL, Greenwood B, and van de Beek D, “Effect of vaccines on bacterial meningitis worldwide,” Lancet, vol. 380, pp. 1703–1711, November 10 2012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

