The Anatomy and Ultrastructure of the Digestive Tract and Salivary Glands of Hishimonus lamellatus (Hemiptera: Cicadellidae)
- PMID: 31268547
- PMCID: PMC6607961
- DOI: 10.1093/jisesa/iez061
The Anatomy and Ultrastructure of the Digestive Tract and Salivary Glands of Hishimonus lamellatus (Hemiptera: Cicadellidae)
Abstract
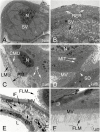
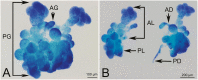
In recent years, we found that Hishimonus lamellatus Cai et Kuoh is a potential vector of jujube witches'-broom phytoplasma. However, little is known about the anatomy and histology of this leafhopper. Here, we examined histology and ultrastructure of the digestive system of H. lamellatus, both by dissecting and by semi- and ultrathin sectioning techniques. We found that the H. lamellatus digestive tract consists of an esophagus, a filter chamber, a conical midgut and midgut loop, Malpighian tubules, an ileum, and a rectum. Furthermore, both the basal region of the filter chamber epithelium and the apical surface of the midgut epithelium have developed microvilli. We also identify the perimicrovillar membrane, which ensheaths the microvilli of midgut loop enterocyte, and the flame-like luminal membrane, which covers the microvilli of the conical midgut epithelium. In addition, H. lamellatus has the principal and accessory salivary glands. Our observations also showed that the endoplasmic reticulum, mitochondria, and secretory granules were all highly abundant in the secretory cells of the principal salivary glands, while the accessory glands consist of only one ovate or elbow-like acinus. We also briefly contrast the structure of the gut of H. lamellatus with those of other leafhopper species. These results intend to offer help for the future study on the histological and subcellular levels of phytopathogen-leafhopper relationships, including transmission barriers and the binding sites of pathogens and other microorganisms within their leafhopper vectors.
Keywords: leafhopper; Malpighian tubule; digestive system; histology; ultrastructure.
© The Author(s) 2019. Published by Oxford University Press on behalf of Entomological Society of America.
Figures







Similar articles
-
Morphology and histology of the digestive system of the vector leafhopper Psammotettix striatus (L.) (Hemiptera: Cicadellidae).Micron. 2012 Jun;43(6):725-38. doi: 10.1016/j.micron.2012.01.004. Epub 2012 Jan 28. Micron. 2012. PMID: 22341338
-
Anatomy and fine structure of the alimentary canal of the spittlebug Lepyronia coleopterata (L.) (Hemiptera: Cercopoidea).Arthropod Struct Dev. 2013 Nov;42(6):521-530. doi: 10.1016/j.asd.2013.04.005. Epub 2013 May 21. Arthropod Struct Dev. 2013. PMID: 23707348
-
Morphological structure of salivary glands, alimentary canal, and Malpighian tubules in adult Eurydema spectabilis Horváth, 1882 (Heteroptera, Pentatomidae).Microsc Res Tech. 2025 Jan;88(1):323-341. doi: 10.1002/jemt.24684. Epub 2024 Oct 2. Microsc Res Tech. 2025. PMID: 39359109 Free PMC article.
-
Multiplex-PCR for Identification of Two Species in Genus Hishimonus (Hemiptera: Cicadellidae) in Jujube Orchards.J Econ Entomol. 2015 Oct;108(5):2443-9. doi: 10.1093/jee/tov191. Epub 2015 Jul 3. J Econ Entomol. 2015. PMID: 26453733
-
Membrane dynamics in insect malpighian tubules.Am J Physiol. 1989 Nov;257(5 Pt 2):R967-72. doi: 10.1152/ajpregu.1989.257.5.R967. Am J Physiol. 1989. PMID: 2686468 Review.
Cited by
-
The cryptonephridial/rectal complex: an evolutionary adaptation for water and ion conservation.Biol Rev Camb Philos Soc. 2025 Apr;100(2):647-671. doi: 10.1111/brv.13156. Epub 2024 Oct 22. Biol Rev Camb Philos Soc. 2025. PMID: 39438273 Free PMC article. Review.
-
Spotlight on the Roles of Whitefly Effectors in Insect-Plant Interactions.Front Plant Sci. 2021 Jul 2;12:661141. doi: 10.3389/fpls.2021.661141. eCollection 2021. Front Plant Sci. 2021. PMID: 34276723 Free PMC article. Review.
-
Peculiar structural features of midgut symbiotic organ in the early development of the stinkbug Plautia stali Scott, 1874 (Hemiptera: Pentatomidae).Naturwissenschaften. 2025 Apr 29;112(3):34. doi: 10.1007/s00114-025-01986-0. Naturwissenschaften. 2025. PMID: 40299062
References
-
- Ammar E. D., Nault L. R., and Rodriguez J. G.. . 1985. Internal morphology and ultrastructure of leafhoppers and planthopper, pp. 127–162. In L. R. Nault and J. G. Rodriguez (eds.), Leafhoppers & planthoppers. John Wiley & Sons, New York.
-
- Ammar e. l.-.D., Gargani D., Lett J. M., and Peterschmitt M.. 2009. Large accumulations of maize streak virus in the filter chamber and midgut cells of the leafhopper vector Cicadulina mbila. Arch. Virol. 154: 255–262. - PubMed
-
- Ammar E., Shatters R. G., and Hall D. G.. . 2011. Localization of Candidatus Liberibacter asiaticus, associated with citrus huanglongbing disease, in its psyllid vector using fluorescence in situ hybridization. J. Phytopathol. 159: 726–734.
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

