Dissociation of natriuresis and diuresis by oxytocin molecular forms in rats
- PMID: 31269062
- PMCID: PMC6608960
- DOI: 10.1371/journal.pone.0219205
Dissociation of natriuresis and diuresis by oxytocin molecular forms in rats
Abstract
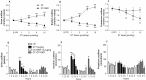
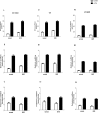
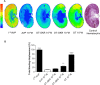
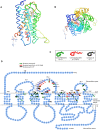
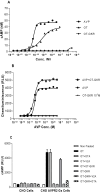
In the rat, oxytocin (OT) produces dose-dependent diuretic and natriuretic responses. Post-translational enzymatic conversion of the OT biosynthetic precursor forms both mature and C-terminally extended peptides. The plasma concentrations of these C-terminally extended peptides (OT-G; OT-GK and OT-GKR) are elevated in newborns and pregnant rats. Intravenous injection of OT-GKR to rats inhibits diuresis, whereas injection of amidated OT stimulates diuresis. Since OT and OT-GKR show different effects on the urine flow, we investigated whether OT-GKR modulates renal action by inhibition of the arginine-vasopressin (AVP) receptor V2 (V2R), the receptor involved in renal water reabsorption. Experiments were carried out in the 8-week-old Wistar rats receiving intravenous (iv) injections of vehicle, OT, OT-GKR or OT+OT-GKR combination. OT (10 μmol/kg) increased urine outflow by 40% (P<0.01) and sodium excretion by 47% (P<0.01). Treatment with OT-GKR (10 μmol/kg) decreased diuresis by 50% (P<0.001), decreased sodium excretion by 50% (P<0.05) and lowered potassium by 42% (P<0.05). OT antagonist (OTA) reduced diuresis and natriuresis exerted by OT, whereas the anti-diuretic effect of OT-GKR was unaffected by OTA. The treatment with V2R antagonist (V2A) in the presence and absence of OT induced diuresis, sodium and potassium outflow. V2A in the presence of OT-GKR only partially increased diuresis and natriuresis. Autoradiography and molecular docking analysis showed potent binding of OT-GKR to V2R. Finally, the release of cAMP from CHO cells overexpressing V2 receptor was induced by low concentration of AVP (EC50:4.2e-011), at higher concentrations of OT (EC50:3.2e-010) and by the highest concentrations of OT-GKR (EC50:1.1e-006). OT-GKR potentiated cAMP release when combined with AVP, but blocked cAMP release when combined with OT. These results suggest that OT-GKR by competing for the OT renal receptor (OTR) and binding to V2R in the kidney, induces anti-diuretic, anti-natriuretic, and anti-kaliuretic effects.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Renal prostaglandins and natriuretic action of oxytocin and vasopressin in rats.J Pharmacol Exp Ther. 1988 Aug;246(2):603-9. J Pharmacol Exp Ther. 1988. PMID: 3165443
-
Natriuretic action of neurohypophysial peptides: effects of agonists and antagonists and implication of natriuretic receptor.J Pharmacol Exp Ther. 1988 Aug;246(2):597-602. J Pharmacol Exp Ther. 1988. PMID: 2969979
-
Molecular pharmacology of human vasopressin receptors.Adv Exp Med Biol. 1998;449:251-76. doi: 10.1007/978-1-4615-4871-3_34. Adv Exp Med Biol. 1998. PMID: 10026814
-
Influence of oxytocin on renal hemodynamics and sodium excretion.Ann N Y Acad Sci. 1993 Jul 22;689:346-62. doi: 10.1111/j.1749-6632.1993.tb55559.x. Ann N Y Acad Sci. 1993. PMID: 8396871 Review.
-
Identification of neurohypophysial hormone receptor domains involved in ligand binding and G protein coupling.Adv Exp Med Biol. 1998;449:371-85. doi: 10.1007/978-1-4615-4871-3_48. Adv Exp Med Biol. 1998. PMID: 10026828 Review.
Cited by
-
The Mechanism of the Solute-Free Water Reabsorption Increase in the Rat Kidney by Oxytocin Saluresis.Dokl Biochem Biophys. 2021 Mar;497(1):95-98. doi: 10.1134/S1607672921020113. Epub 2021 Mar 5. Dokl Biochem Biophys. 2021. PMID: 33666805 Free PMC article.
-
Hematological changes, oxidative stress assessment, and dysregulation of aquaporin-3 channel, prolactin, and oxytocin receptors in kidneys of lactating Wistar rats treated with monosodium glutamate.Naunyn Schmiedebergs Arch Pharmacol. 2024 Aug;397(8):6213-6229. doi: 10.1007/s00210-024-03008-8. Epub 2024 Mar 6. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38446217
-
Partial nephrogenic diabetes insipidus with a novel arginine vasopressin receptor 2 gene variant.Clin Pediatr Endocrinol. 2022;31(1):44-49. doi: 10.1297/cpe.2021-0029. Epub 2021 Nov 1. Clin Pediatr Endocrinol. 2022. PMID: 35002068 Free PMC article.
-
Nephrogenic diabetes insipidus caused by a novel missense variant (p.S127Y) in the AVPR2 gene.Clin Pediatr Endocrinol. 2021;30(2):115-118. doi: 10.1297/cpe.30.115. Epub 2021 Apr 3. Clin Pediatr Endocrinol. 2021. PMID: 33867673 Free PMC article. No abstract available.
-
The Role of Oxytocin in Cardiovascular Protection.Front Psychol. 2020 Aug 25;11:2139. doi: 10.3389/fpsyg.2020.02139. eCollection 2020. Front Psychol. 2020. PMID: 32982875 Free PMC article. Review.
References
-
- Kocyigit UM, Taskiran AS, Taslimi P, Yokus A, Temel Y, Gulcin I. Inhibitory effects of oxytocin and oxytocin receptor antagonist atosiban on the activities of carbonic anhydrase and acetylcholinesterase enzymes in the liver and kidney tissues of rats. J Biochem Mol Toxicol. 2017;31(11). Epub 2017/11/09. 10.1002/jbt.21972 . - DOI - PubMed
-
- Tribollet E, Barberis C, Dreifuss JJ, Jard S. Autoradiographic localization of vasopressin and oxytocin binding sites in rat kidney. Kidney international. 1988;33(5):959–65. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

