Regulation of the Bone Vascular Network is Sexually Dimorphic
- PMID: 31269275
- PMCID: PMC6899569
- DOI: 10.1002/jbmr.3825
Regulation of the Bone Vascular Network is Sexually Dimorphic
Abstract
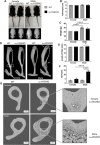
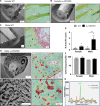
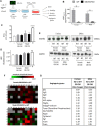
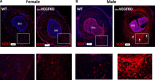
Osteoblast (OB) lineage cells are an important source of vascular endothelial growth factor (VEGF), which is critical for bone growth and repair. During bone development, pubertal differences in males and females exist, but little is known about whether VEGF signaling contributes to skeletal sexual dimorphism. We have found that in mice, conditional disruption of VEGF in osteocalcin-expressing cells (OcnVEGFKO) exerts a divergent influence on morphological, cellular, and whole bone properties between sexes. Furthermore, we describe an underlying sexual divergence in VEGF signaling in OB cultures in vitro independent of circulating sex hormones. High-resolution synchrotron computed tomography and backscattered scanning electron microscopy revealed, in males, extensive unmineralized osteoid encasing enlarged blood vessel canals and osteocyte lacunae in cortical bone after VEGF deletion, which contributed to increased porosity. VEGF was deleted in male and female long bone-derived OBs (OBVEGKO) in vitro and Raman spectroscopic analyses of mineral and matrix repertoires highlighted differences between male and female OBVEGFKO cells, with increased immature phosphate species prevalent in male OBVEGFKO cultures versus wild type (WT). Further sexual dimorphism was observed in bone marrow endothelial cell gene expression in vitro after VEGF deletion and in sclerostin protein expression, which was increased in male OcnVEGFKO bones versus WT. The impact of altered OB matrix composition after VEGF deletion on whole bone geometry was assessed between sexes, although significant differences between OcnVEGFKO and WT were identified only in females. Our results suggest that bone-derived VEGF regulates matrix mineralization and vascularization distinctly in males and females, which results in divergent physical bone traits.
Keywords: BONE QCT/MICROCT; GENETIC ANIMAL MODELS; MATRIX MINERALIZATION; OSTEOBLASTS; PRECLINICAL STUDIES.
© 2019 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals, Inc.
Figures



Similar articles
-
Multiscale molecular profiling of pathological bone resolves sexually dimorphic control of extracellular matrix composition.Dis Model Mech. 2021 Mar 1;14(3):dmm048116. doi: 10.1242/dmm.048116. Epub 2021 Mar 17. Dis Model Mech. 2021. PMID: 33563616 Free PMC article.
-
Sexually dimorphic effects of prenatal alcohol exposure on the murine skeleton.Biol Sex Differ. 2024 Jun 18;15(1):51. doi: 10.1186/s13293-024-00626-y. Biol Sex Differ. 2024. PMID: 38890762 Free PMC article.
-
CYR61/CCN1 Regulates Sclerostin Levels and Bone Maintenance.J Bone Miner Res. 2018 Jun;33(6):1076-1089. doi: 10.1002/jbmr.3394. Epub 2018 Mar 5. J Bone Miner Res. 2018. PMID: 29351359 Free PMC article.
-
IGF-I Signaling in Osterix-Expressing Cells Regulates Secondary Ossification Center Formation, Growth Plate Maturation, and Metaphyseal Formation During Postnatal Bone Development.J Bone Miner Res. 2015 Dec;30(12):2239-48. doi: 10.1002/jbmr.2563. Epub 2015 Jul 29. J Bone Miner Res. 2015. PMID: 26011431 Free PMC article.
-
Vascular endothelial growth factor control mechanisms in skeletal growth and repair.Dev Dyn. 2017 Apr;246(4):227-234. doi: 10.1002/dvdy.24463. Epub 2016 Dec 29. Dev Dyn. 2017. PMID: 27750398 Free PMC article. Review.
Cited by
-
Temporal and Quantitative Transcriptomic Differences Define Sexual Dimorphism in Murine Postnatal Bone Aging.JBMR Plus. 2021 Dec 10;6(2):e10579. doi: 10.1002/jbm4.10579. eCollection 2022 Feb. JBMR Plus. 2021. PMID: 35229061 Free PMC article.
-
A robust, semi-automated approach for counting cementum increments imaged with synchrotron X-ray computed tomography.PLoS One. 2021 Nov 4;16(11):e0249743. doi: 10.1371/journal.pone.0249743. eCollection 2021. PLoS One. 2021. PMID: 34735460 Free PMC article.
-
Multiscale molecular profiling of pathological bone resolves sexually dimorphic control of extracellular matrix composition.Dis Model Mech. 2021 Mar 1;14(3):dmm048116. doi: 10.1242/dmm.048116. Epub 2021 Mar 17. Dis Model Mech. 2021. PMID: 33563616 Free PMC article.
-
Sex-Based Difference in Bone Healing: A Review of Recent Pre-clinical Literature.Curr Rev Musculoskelet Med. 2022 Dec;15(6):651-658. doi: 10.1007/s12178-022-09803-1. Epub 2022 Nov 15. Curr Rev Musculoskelet Med. 2022. PMID: 36378466 Free PMC article. Review.
-
Sexually dimorphic effects of prenatal alcohol exposure on the murine skeleton.Biol Sex Differ. 2024 Jun 18;15(1):51. doi: 10.1186/s13293-024-00626-y. Biol Sex Differ. 2024. PMID: 38890762 Free PMC article.
References
-
- Liu Y, Olsen BR. Distinct VEGF functions during bone development and homeostasis. Arch Immunol Ther Exp (Warsz). 2014;62:363–8. - PubMed
-
- Kanczler JM, Oreffo RO. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater. 2008;15:100–14. - PubMed
-
- Clarkin CE, Gerstenfeld LC. VEGF and bone cell signaling: an essential vessel for communication? Cell Biochem Funct. 2013;31:1–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

