Neuronal Soma-Derived Degradative Lysosomes Are Continuously Delivered to Distal Axons to Maintain Local Degradation Capacity
- PMID: 31269450
- PMCID: PMC6696943
- DOI: 10.1016/j.celrep.2019.06.013
Neuronal Soma-Derived Degradative Lysosomes Are Continuously Delivered to Distal Axons to Maintain Local Degradation Capacity
Abstract
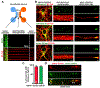
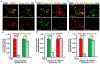
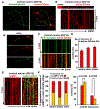
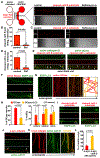
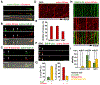
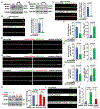
Neurons face the challenge of maintaining cellular homeostasis through lysosomal degradation. While enzymatically active degradative lysosomes are enriched in the soma, their axonal trafficking and positioning and impact on axonal physiology remain elusive. Here, we characterized axon-targeted delivery of degradative lysosomes by applying fluorescent probes that selectively label active forms of lysosomal cathepsins D, B, L, and GCase. By time-lapse imaging of cortical neurons in microfluidic devices and standard dishes, we reveal that soma-derived degradative lysosomes rapidly influx into distal axons and target to autophagosomes and Parkinson disease-related α-synuclein cargos for local degradation. Impairing lysosome axonal delivery induces an aberrant accumulation of autophagosomes and α-synuclein cargos in distal axons. Our study demonstrates that the axon is an active compartment for local degradation and reveals fundamental aspects of axonal lysosomal delivery and maintenance. Our work establishes a foundation for investigations into axonal lysosome trafficking and functionality in neurodegenerative diseases.
Keywords: active lysosomal hydrolase; autophagic stress; autophagosome; axonal transport; cathepsin; degradative lysosome; lysosomal trafficking; α-synuclein.
Published by Elsevier Inc.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing financial interests.
Figures

References
-
- Bagshaw RD, Callahan JW, and Mahuran DJ (2006). The Arf-family protein, Arl8b, is involved in the spatial distribution of lysosomes. Biochem. Biophys. Res. Commun 344, 1186–1191. - PubMed
-
- Beard H, Hassiotis S, Gai WP, Parkinson-Lawrence E, Hopwood JJ, and Hemsley KM (2017). Axonal dystrophy in the brain of mice with Sanfilippo syndrome. Exp. Neurol 295, 243–255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

