Long Noncoding RNAs and Stress Response in the Nucleolus
- PMID: 31269716
- PMCID: PMC6678565
- DOI: 10.3390/cells8070668
Long Noncoding RNAs and Stress Response in the Nucleolus
Abstract
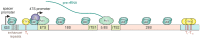
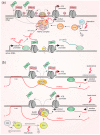
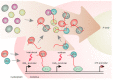
Long noncoding RNAs (lncRNAs) perform diverse functions in the regulation of cellular processes. Here we consider a variety of lncRNAs found in the ribosome production center, the nucleolus, and focus on their role in the response to environmental stressors. Nucleolar lncRNAs ensure stress adaptation by cessation of resource-intensive ribosomal RNA (rRNA) synthesis and by inducing the massive sequestration of proteins within the nucleolus. Different cell states like quiescence and cancer are also controlled by specific lncRNAs in the nucleolus. Taken together, recent findings allow us to consider lncRNAs as multifunctional regulators of nucleolar activities, which are responsive to various physiological conditions.
Keywords: PAPAS; SLERT; chromatin; intergenic spacers; lncRNA; noncoding RNA; nucleolus; pRNA; ribosomal RNA; stress.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Long non-coding RNAs in the nucleolus: Biogenesis, regulation, and function.Curr Opin Struct Biol. 2024 Aug;87:102866. doi: 10.1016/j.sbi.2024.102866. Epub 2024 Jun 22. Curr Opin Struct Biol. 2024. PMID: 38909586 Review.
-
Stress-Induced Evolution of the Nucleolus: The Role of Ribosomal Intergenic Spacer (rIGS) Transcripts.Biomolecules. 2024 Oct 20;14(10):1333. doi: 10.3390/biom14101333. Biomolecules. 2024. PMID: 39456266 Free PMC article. Review.
-
Non-coding RNAs at the Eukaryotic rDNA Locus: RNA-DNA Hybrids and Beyond.J Mol Biol. 2020 Jul 10;432(15):4287-4304. doi: 10.1016/j.jmb.2020.05.011. Epub 2020 May 21. J Mol Biol. 2020. PMID: 32446803 Review.
-
Regulatory roles of nucleolus organizer region-derived long non-coding RNAs.Mamm Genome. 2022 Jun;33(2):402-411. doi: 10.1007/s00335-021-09906-z. Epub 2021 Aug 26. Mamm Genome. 2022. PMID: 34436664 Free PMC article. Review.
-
A nucleolar long "non-coding" RNA encodes a novel protein that functions in response to stress.Proc Natl Acad Sci U S A. 2023 Feb 28;120(9):e2221109120. doi: 10.1073/pnas.2221109120. Epub 2023 Feb 22. Proc Natl Acad Sci U S A. 2023. PMID: 36812203 Free PMC article.
Cited by
-
Beyond rRNA: nucleolar transcription generates a complex network of RNAs with multiple roles in maintaining cellular homeostasis.Genes Dev. 2022 Aug 1;36(15-16):876-886. doi: 10.1101/gad.349969.122. Genes Dev. 2022. PMID: 36207140 Free PMC article. Review.
-
Functions of RNAi Pathways in Ribosomal RNA Regulation.Noncoding RNA. 2024 Mar 29;10(2):19. doi: 10.3390/ncrna10020019. Noncoding RNA. 2024. PMID: 38668377 Free PMC article. Review.
-
Long Noncoding RNA VPS9D1-AS1 Sequesters microRNA-525-5p to Promote the Oncogenicity of Colorectal Cancer Cells by Upregulating HMGA1.Cancer Manag Res. 2020 Oct 9;12:9915-9928. doi: 10.2147/CMAR.S273687. eCollection 2020. Cancer Manag Res. 2020. Retraction in: Cancer Manag Res. 2023 Jul 13;15:685-686. doi: 10.2147/CMAR.S430213. PMID: 33116849 Free PMC article. Retracted.
-
Parallel Proteomic and Transcriptomic Microenvironment Mapping (μMap) of Nuclear Condensates in Living Cells.J Am Chem Soc. 2025 Jan 8;147(1):488-497. doi: 10.1021/jacs.4c11612. Epub 2024 Dec 21. J Am Chem Soc. 2025. PMID: 39707993 Free PMC article.
-
Long Non-Coding RNA LINC00491 Contributes to the Malignancy of Non-Small-Cell Lung Cancer via Competitively Binding to microRNA-324-5p and Thereby Increasing Specificity Protein 1 Expression.Cancer Manag Res. 2020 Aug 6;12:6779-6793. doi: 10.2147/CMAR.S264681. eCollection 2020. Cancer Manag Res. 2020. Retraction in: Cancer Manag Res. 2021 Sep 29;13:7505-7506. doi: 10.2147/CMAR.S341520. PMID: 32821159 Free PMC article. Retracted.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

