Preeclampsia: The Relationship between Uterine Artery Blood Flow and Trophoblast Function
- PMID: 31269775
- PMCID: PMC6651116
- DOI: 10.3390/ijms20133263
Preeclampsia: The Relationship between Uterine Artery Blood Flow and Trophoblast Function
Abstract
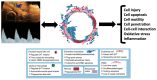
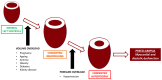
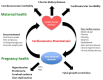
Maternal uterine artery blood flow is critical to maintaining the intrauterine environment, permitting normal placental function, and supporting fetal growth. It has long been believed that inadequate transformation of the maternal uterine vasculature is a consequence of primary defective trophoblast invasion and leads to the development of preeclampsia. That early pregnancy maternal uterine artery perfusion is strongly associated with placental cellular function and behaviour has always been interpreted in this context. Consistently observed changes in pre-conceptual maternal and uterine artery blood flow, abdominal pregnancy implantation, and late pregnancy have been challenging this concept, and suggest that abnormal placental perfusion may result in trophoblast impairment, rather than the other way round. This review focuses on evidence that maternal cardiovascular function plays a significant role in the pathophysiology of preeclampsia.
Keywords: maternal cardiovascular system; preeclampsia; uterine artery.
Conflict of interest statement
The authors declare no conflict of interest. The funder had no role in the design of the study and in the writing of the manuscript.
Figures

References
-
- Cnossen J., Morris R., ter Riet G., Mol B.W., van der Post J.A., Coomarasamy A., Zwinderman A.H., Robson S.C., Bindels P.J., Kleijnen J., et al. Use of uterine artery Doppler ultrasonography to predict pre-eclampsia and intrauterine growth restriction: a systematic review and bivariable meta-analysis. CMAJ. 2008;178:1–11. doi: 10.1503/cmaj.070430. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

