Effects of Air Pollution on Lung Innate Lymphoid Cells: Review of In Vitro and In Vivo Experimental Studies
- PMID: 31269777
- PMCID: PMC6650824
- DOI: 10.3390/ijerph16132347
Effects of Air Pollution on Lung Innate Lymphoid Cells: Review of In Vitro and In Vivo Experimental Studies
Abstract
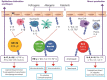
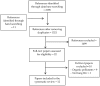
Outdoor air pollution is associated with respiratory infections and allergies, yet the role of innate lymphoid cells (ILCs) in pathogen containment and airway hyperresponsiveness relevant to effects of air pollutants on ILCs is poorly understood. We conducted a systematic review to evaluate the available evidence on the effect of outdoor air pollutants on the lung type 1 (ILC1) and type 2 ILCs (ILC2) subsets. We searched five electronic databases (up to Dec 2018) for studies on the effect of carbon monoxide (CO), sulfur dioxide (SO2), nitrogen dioxide (NO2), diesel exhaust particles (DEP), ozone (O3), and particulate matter (PM) on respiratory ILCs. Of 2209 identified citations, 22 full-text papers were assessed for eligibility, and 12 articles describing experimental studies performed in murine strains (9) and on human blood cells (3) were finally selected. Overall, these studies showed that exposure to PM, DEP, and high doses of O3 resulted in a reduction of interferon gamma (IFN-γ) production and cytotoxicity of ILC1. These pollutants and carbon nanotubes stimulate lung ILC2s, produce high levels of interleukin (IL)-5 and IL-13, and induce airway hyperresponsiveness. These findings highlight potential mechanisms by which human ILCs react to air pollution that increase the susceptibility to infections and allergies.
Keywords: ILC; air pollutants; airway hyperresponsiveness; lung innate lymphoid cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

