Antigen structure affects cellular routing through DC-SIGN
- PMID: 31270240
- PMCID: PMC6660738
- DOI: 10.1073/pnas.1820165116
Antigen structure affects cellular routing through DC-SIGN
Abstract
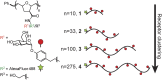
Dendritic cell (DC) lectins mediate the recognition, uptake, and processing of antigens, but they can also be coopted by pathogens for infection. These distinct activities depend upon the routing of antigens within the cell. Antigens directed to endosomal compartments are degraded, and the peptides are presented on major histocompatibility complex class II molecules, thereby promoting immunity. Alternatively, HIV-1 can avoid degradation, as virus engagement with C-type lectin receptors (CLRs), such as DC-SIGN (DC-specific ICAM-3-grabbing nonintegrin) results in trafficking to surface-accessible invaginated pockets. This process appears to enable infection of T cells in trans We sought to explore whether antigen fate upon CLR-mediated internalization was affected by antigen physical properties. To this end, we employed the ring-opening metathesis polymerization to generate glycopolymers that each display multiple copies of mannoside ligand for DC-SIGN, yet differ in length and size. The rate and extent of glycopolymer internalization depended upon polymer structure-longer polymers were internalized more rapidly and more efficiently than were shorter polymers. The trafficking, however, did not differ, and both short and longer polymers colocalized with transferrin-labeled early endosomes. To explore how DC-SIGN directs larger particles, such as pathogens, we induced aggregation of the polymers to access particulate antigens. Strikingly, these particulate antigens were diverted to the invaginated pockets that harbor HIV-1. Thus, antigen structure has a dramatic effect on DC-SIGN-mediated uptake and trafficking. These findings have consequences for the design of synthetic vaccines. Additionally, the results suggest strategies for targeting DC reservoirs that harbor viral pathogens.
Keywords: C-type lectin; HIV; antigen; endocytosis; polymer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Figdor C. G., van Kooyk Y., Adema G. J., C-type lectin receptors on dendritic cells and Langerhans cells. Nat. Rev. Immunol. 2, 77–84 (2002). - PubMed
-
- Weis W. I., Kahn R., Fourme R., Drickamer K., Hendrickson W. A., Structure of the calcium-dependent lectin domain from a rat mannose-binding protein determined by MAD phasing. Science 254, 1608–1615 (1991). - PubMed
-
- Robinson M. J., Sancho D., Slack E. C., LeibundGut-Landmann S., Reis e Sousa C., Myeloid C-type lectins in innate immunity. Nat. Immunol. 7, 1258–1265 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

