JNK and cardiometabolic dysfunction
- PMID: 31270248
- PMCID: PMC6639461
- DOI: 10.1042/BSR20190267
JNK and cardiometabolic dysfunction
Abstract
Cardiometabolic syndrome (CMS) describes the cluster of metabolic and cardiovascular diseases that are generally characterized by impaired glucose tolerance, intra-abdominal adiposity, dyslipidemia, and hypertension. CMS currently affects more than 25% of the world's population and the rates of diseases are rapidly rising. These CMS conditions represent critical risk factors for cardiovascular diseases including atherosclerosis, heart failure, myocardial infarction, and peripheral artery disease (PAD). Therefore, it is imperative to elucidate the underlying signaling involved in disease onset and progression. The c-Jun N-terminal Kinases (JNKs) are a family of stress signaling kinases that have been recently indicated in CMS. The purpose of this review is to examine the in vivo implications of JNK as a potential therapeutic target for CMS. As the constellation of diseases associated with CMS are complex and involve multiple tissues and environmental triggers, carefully examining what is known about the JNK pathway will be important for specificity in treatment strategies.
Keywords: Signaling; cardiovascular disease; metabolic regulation.
© 2019 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


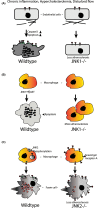
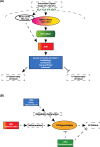

References
-
- Kyriakis J.M. and Avruch J. (1990) pp54 microtubule-associated protein 2 kinase. A novel serine/threonine protein kinase regulated by phosphorylation and stimulated by poly-L-lysine. J. Biol. Chem. 265, 17355–17363 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

