Down-regulated microRNA-30b-3p inhibits proliferation, invasion and migration of glioma cells via inactivation of the AKT signaling pathway by up-regulating RECK
- PMID: 31270250
- PMCID: PMC6692569
- DOI: 10.1042/BSR20182226
Down-regulated microRNA-30b-3p inhibits proliferation, invasion and migration of glioma cells via inactivation of the AKT signaling pathway by up-regulating RECK
Retraction in
-
Retraction: Downregulated microRNA-30b-3p inhibits proliferation, invasion and migration of glioma cells via inactivation of the AKT signaling pathway by upregulating RECK.Biosci Rep. 2024 May 29;44(5):BSR-2018-2226_RET. doi: 10.1042/BSR-2018-2226_RET. Biosci Rep. 2024. PMID: 38743460 Free PMC article. No abstract available.
Abstract
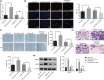
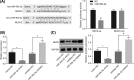
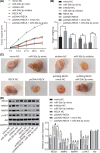
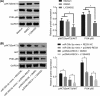
microRNAs (miRNAs) have been found to affect various cancers, and expression of numerous miRNAs is revealed in glioma. However, the role of microRNA-30b-3p (miR-30b-3p) in glioma remains elusive. Therefore, the present study aims to explore the specific mechanism by which miR-30b-3p influence the development of glioma in relation to the AKT signaling pathway. First, glioma cell lines were collected with miR-30b-3p and reversion-inducing cysteine-rich protein with kazal motifs (RECK) expression measured. The functional role of miR-30b-3p and RECK in glioma was determined via gain- and loss-of-function approaches. Subsequently, the expression of invasion- and migration-related factors (MMP-2 and MMP-9) and the AKT signaling pathway-related factors (AKT, p-AKT and PI3K-p85) was detected. Moreover, in vivo experiments were also conducted to investigate how miR-30b-3p influences in vivo tumorigenesis. The results showed that miR-30b-3p was up-regulated and RECK was down-regulated in glioma. RECK was a target gene of miR-30b-3p. Decreased miR-30b-3p and overexpressed RECK led to decreased expression of MMP-2, MMP-9 and p-AKT. Overexpressed RECK and LY294002 could decrease p-AKT and PI3K-p85 expression accompanied with unchanged expression of total protein of AKT. Additionally, proliferation, migration and invasion of glioma cells and tumor formation in nude mice were repressed owing to reduced expression of miR-30b-3p or elevated expression of RECK. In summary, miR-30b-3p inhibition suppresses metastasis of glioma cells by inactivating the AKT signaling pathway via RECK up-regulation, providing a new target for glioma treatment.
Keywords: AKT signaling pathway; Glioma; Invasion; MicroRNA-30b-3p; Migration; Reversion-inducing cysteine-rich protein with kazal motifs.
© 2019 The Author(s).
Conflict of interest statement
The present study was carried out strictly conforming to the recommendations in the Guide for the Care and Use of Laboratory Animals, and was approved by the Animal Ethics Committee of The First Affiliated Hospital of Nanchang University.
The authors declare that there are no competing interests associated with the manuscript.
Figures



References
-
- Seliger C., Luber C., Gerken M., Schaertl J., Proescholdt M., Riemenschneider M.J.. et al. (2018) Use of metformin and survival of patients with high-grade glioma. Int. J. Cancer, 144, 273–280 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

