CFTR-PTEN-dependent mitochondrial metabolic dysfunction promotes Pseudomonas aeruginosa airway infection
- PMID: 31270271
- PMCID: PMC6784538
- DOI: 10.1126/scitranslmed.aav4634
CFTR-PTEN-dependent mitochondrial metabolic dysfunction promotes Pseudomonas aeruginosa airway infection
Abstract
Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) is a tumor suppressor best known for regulating cell proliferation and metabolism. PTEN forms a complex with the cystic fibrosis (CF) transmembrane conductance regulator (CFTR) at the plasma membrane, and this complex is known to be functionally impaired in CF. Here, we demonstrated that the combined effect of PTEN and CFTR dysfunction stimulates mitochondrial activity, resulting in excessive release of succinate and reactive oxygen species. This environment promoted the colonization of the airway by Pseudomonas aeruginosa, bacteria that preferentially metabolize succinate, and stimulated an anti-inflammatory host response dominated by immune-responsive gene 1 (IRG1) and itaconate. The recruitment of myeloid cells induced by these strains was inefficient in clearing the infection and increased numbers of phagocytes accumulated under CFTR-PTEN axis dysfunction. This central metabolic defect in mitochondrial function due to impaired PTEN activity contributes to P. aeruginosa infection in CF.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures
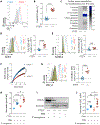




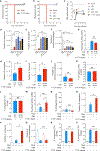
Comment in
-
Succinate links mitochondria to deadly bacteria in cystic fibrosis.Ann Transl Med. 2019 Dec;7(Suppl 8):S263. doi: 10.21037/atm.2019.12.49. Ann Transl Med. 2019. PMID: 32015982 Free PMC article. No abstract available.
References
-
- Luciani A, Villella VR, Esposito S, Brunetti-Pierri N, Medina D, Settembre C, Gavina M, Pulze L, Giardino I, Pettoello-Mantovani M, D’Apolito M, Guido S, Masliah E, Spencer B, Quaratino S, Raia V, Ballabio A, Maiuri L, Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat. Cell Biol 12, 863–875 (2010). - PubMed
-
- Elborn JS, Cystic fibrosis. Lancet 388, 2519–2531 (2016). - PubMed
-
- Blouin M-J, Zhao Y, Zakikhani M, Algire C, Piura E, Pollak M, Loss of function of PTEN alters the relationship between glucose concentration and cell proliferation, increases glycolysis, and sensitizes cells to 2-deoxyglucose. Cancer Lett 289, 246–253 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

