The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells
- PMID: 31270272
- PMCID: PMC7523030
- DOI: 10.1126/scitranslmed.aau5907
The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells
Abstract
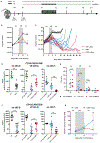
Immunotherapy with chimeric antigen receptor (CAR)-engineered T cells can be effective against advanced malignancies. CAR T cells are "living drugs" that require technologies to enable physicians (and patients) to maintain control over the infused cell product. Here, we demonstrate that the tyrosine kinase inhibitor dasatinib interferes with the lymphocyte-specific protein tyrosine kinase (LCK) and thereby inhibits phosphorylation of CD3ζ and ζ-chain of T cell receptor-associated protein kinase 70 kDa (ZAP70), ablating signaling in CAR constructs containing either CD28_CD3ζ or 4-1BB_CD3ζ activation modules. As a consequence, dasatinib induces a function-off state in CD8+ and CD4+ CAR T cells that is of immediate onset and can be sustained for several days without affecting T cell viability. We show that treatment with dasatinib halts cytolytic activity, cytokine production, and proliferation of CAR T cells in vitro and in vivo. The dose of dasatinib can be titrated to achieve partial or complete inhibition of CAR T cell function. Upon discontinuation of dasatinib, the inhibitory effect is rapidly and completely reversed, and CAR T cells resume their antitumor function. The favorable pharmacodynamic attributes of dasatinib can be exploited to steer the activity of CAR T cells in "function-on-off-on" sequences in real time. In a mouse model of cytokine release syndrome (CRS), we demonstrated that a short treatment course of dasatinib, administered early after CAR T cell infusion, protects a proportion of mice from otherwise fatal CRS. Our data introduce dasatinib as a broadly applicable pharmacologic on/off switch for CAR T cells.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures


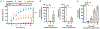
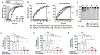

Comment in
-
TKI-based on/off switch for CAR T cells.Nat Rev Clin Oncol. 2019 Sep;16(9):528. doi: 10.1038/s41571-019-0258-5. Nat Rev Clin Oncol. 2019. PMID: 31312040 No abstract available.
-
CAR T Cells: Hitting the Pause Button.Cancer Discov. 2019 Sep;9(9):1149-1150. doi: 10.1158/2159-8290.CD-NB2019-086. Epub 2019 Jul 24. Cancer Discov. 2019. PMID: 31340935
-
Steering CARs in the right direction.Nat Rev Cancer. 2019 Sep;19(9):487. doi: 10.1038/s41568-019-0189-6. Nat Rev Cancer. 2019. PMID: 31371792 No abstract available.
References
-
- Turtle CJ, Hanafi L-A, Berger C, Hudecek M, Pender B, Robinson E, Hawkins R, Chaney C, Cherian S, Chen X, Soma L, Wood B, Li D, Heimfeld S, Riddell SR, Maloney DG, Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells., Sci. Transl. Med 8, 355ra116 (2016). - PMC - PubMed
-
- Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, Komanduri KV, Lin Y, Jain N, Daver N, Westin J, Gulbis AM, Loghin ME, de Groot JF, Adkins S, Davis SE, Rezvani K, Hwu P, Shpall EJ, Chimeric antigen receptor T-cell therapy - assessment and management of toxicities., Nat. Rev. Clin. Oncol 15, 47–62 (2018). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

