Agonism of CD11b reprograms innate immunity to sensitize pancreatic cancer to immunotherapies
- PMID: 31270275
- PMCID: PMC7197026
- DOI: 10.1126/scitranslmed.aau9240
Agonism of CD11b reprograms innate immunity to sensitize pancreatic cancer to immunotherapies
Abstract
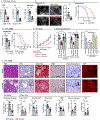
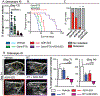
Although checkpoint immunotherapies have revolutionized the treatment of cancer, not all tumor types have seen substantial benefit. Pancreatic ductal adenocarcinoma (PDAC) is a highly lethal malignancy in which very limited responses to immunotherapy have been observed. Extensive immunosuppressive myeloid cell infiltration in PDAC tissues has been postulated as a major mechanism of resistance to immunotherapy. Strategies concomitantly targeting monocyte or granulocyte trafficking or macrophage survival, in combination with checkpoint immunotherapies, have shown promise in preclinical studies, and these studies have transitioned into ongoing clinical trials for the treatment of pancreatic and other cancer types. However, compensatory actions by untargeted monocytes, granulocytes, and/or tissue resident macrophages may limit the therapeutic efficacy of such strategies. CD11b/CD18 is an integrin molecule that is highly expressed on the cell surface of these myeloid cell subsets and plays an important role in their trafficking and cellular functions in inflamed tissues. Here, we demonstrate that the partial activation of CD11b by a small-molecule agonist (ADH-503) leads to the repolarization of tumor-associated macrophages, reduction in the number of tumor-infiltrating immunosuppressive myeloid cells, and enhanced dendritic cell responses. These actions, in turn, improve antitumor T cell immunity and render checkpoint inhibitors effective in previously unresponsive PDAC models. These data demonstrate that molecular agonism of CD11b reprograms immunosuppressive myeloid cell responses and potentially bypasses the limitations of current clinical strategies to overcome resistance to immunotherapy.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Competing interests: VG and DGD have competing financial interest related to ADH-503. VG has partial ownership of Adhaere Pharmaceuticals and DGD is a scientific advisory board member.
Figures





Comment in
-
CD11b agonism overcomes PDAC immunotherapy resistance.Nat Rev Gastroenterol Hepatol. 2019 Sep;16(9):514. doi: 10.1038/s41575-019-0191-1. Nat Rev Gastroenterol Hepatol. 2019. PMID: 31332304 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

