Conditional loss of ERK1 and ERK2 results in abnormal placentation and delayed parturition in the mouse
- PMID: 31270345
- PMCID: PMC6610138
- DOI: 10.1038/s41598-019-45997-0
Conditional loss of ERK1 and ERK2 results in abnormal placentation and delayed parturition in the mouse
Abstract
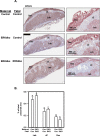
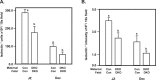
Extracellular-signal-regulated kinases (ERK) 1 and 2 regulate many aspects of the hypothalamic-pituitary-gonadal axis. We sought to understand the role of ERK1/2 signaling in cells expressing a Cre allele regulated by the endogenous GnRHR promoter (GRIC-ERKdko). Adult female GRIC-ERKdko mice were hypogonadotropic and anovulatory. Gonadotropin administration and mating led to pregnancy in one-third of the ERKdko females. Litters from ERKdko females and pup weights were reduced coincident with delayed parturition and 100% neonatal mortality. Based on this, we examined Cre expression in implantation sites as a potential mechanism. GnRHR mRNA levels at e10.5 and e12.5 were comparable to pituitary levels from adult female mice at proestrus and GnRHR mRNA in decidua was enriched compared to whole implantation site. In vivo studies confirmed recombination in decidua, and GRIC-ERKdko placentas showed reduced ERK2 expression. Histopathology revealed abnormalities in placental architecture in the GRIC-ERKdko animals. Regions of apoptosis at the decidual/uterine interface at e18.5 were observed in control animals but apoptotic tone in these regions was reduced in ERKdko animals. These studies support a potential model of ERK-dependent signaling within the implantation site leading to loss of placental architecture and mis-regulation of apoptotic events at parturition occurring coincident with prolonged gestation and neonatal mortality.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

