Functional and metabolic impairment in cigarette smoke-exposed macrophages is tied to oxidative stress
- PMID: 31270372
- PMCID: PMC6610132
- DOI: 10.1038/s41598-019-46045-7
Functional and metabolic impairment in cigarette smoke-exposed macrophages is tied to oxidative stress
Abstract
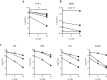
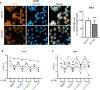
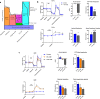
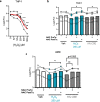
Cigarette smoke inhalation exposes the respiratory system to thousands of potentially toxic substances and causes chronic obstructive pulmonary disease (COPD). COPD is characterized by cycles of inflammation and infection with a dysregulated immune response contributing to disease progression. While smoking cessation can slow the damage in COPD, lung immunity remains impaired. Alveolar macrophages (AMΦ) are innate immune cells strategically poised at the interface between lungs, respiratory pathogens, and environmental toxins including cigarette smoke. We studied the effects of cigarette smoke on model THP-1 and peripheral blood monocyte derived macrophages, and discovered a marked inhibition of bacterial phagocytosis which was replicated in primary human AMΦ. Cigarette smoke decreased AMΦ cystic fibrosis transmembrane conductance regulator (CFTR) expression, previously shown to be integral to phagocytosis. In contrast to cystic fibrosis macrophages, smoke-exposed THP-1 and AMΦ failed to augment phagocytosis in the presence of CFTR modulators. Cigarette smoke also inhibited THP-1 and AMΦ mitochondrial respiration while inducing glycolysis and reactive oxygen species. These effects were mitigated by the free radical scavenger N-acetylcysteine, which also reverted phagocytosis to baseline levels. Collectively these results implicate metabolic dysfunction as a key factor in the toxicity of cigarette smoke to AMΦ, and illuminate avenues of potential intervention.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Pappas RS, Gray N, Gonzalez-Jimenez N, Fresquez M, Watson CH. Triple Quad-ICP-MS Measurement of Toxic Metals in Mainstream Cigarette Smoke from Spectrum Research Cigarettes. J. Anal. Toxicol. 2016;40:43–48. - PubMed
-
- WHO | The global burden of disease: 2004 update. WHO Available at, http://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/. (Accessed: 14th April 2018).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

