Programmable bacteria induce durable tumor regression and systemic antitumor immunity
- PMID: 31270504
- PMCID: PMC6688650
- DOI: 10.1038/s41591-019-0498-z
Programmable bacteria induce durable tumor regression and systemic antitumor immunity
Abstract
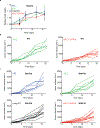
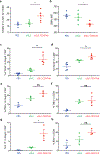
Synthetic biology is driving a new era of medicine through the genetic programming of living cells1,2. This transformative approach allows for the creation of engineered systems that intelligently sense and respond to diverse environments, ultimately adding specificity and efficacy that extends beyond the capabilities of molecular-based therapeutics3-6. One particular area of focus has been the engineering of bacteria as therapeutic delivery systems to selectively release therapeutic payloads in vivo7-11. Here we engineered a non-pathogenic Escherichia coli strain to specifically lyse within the tumor microenvironment and release an encoded nanobody antagonist of CD47 (CD47nb)12, an anti-phagocytic receptor that is commonly overexpressed in several human cancer types13,14. We show that delivery of CD47nb by tumor-colonizing bacteria increases activation of tumor-infiltrating T cells, stimulates rapid tumor regression, prevents metastasis and leads to long-term survival in a syngeneic tumor model in mice. Moreover, we report that local injection of CD47nb-expressing bacteria stimulates systemic tumor-antigen-specific immune responses that reduce the growth of untreated tumors, providing proof-of-concept for an abscopal effect induced by an engineered bacterial immunotherapy. Thus, engineered bacteria may be used for safe and local delivery of immunotherapeutic payloads leading to systemic antitumor immunity.
Conflict of interest statement
COMPETING INTERESTS STATEMENT
S.C., N.A. and T.D. have filed a provisional patent application with the US Patent and Trademark Office (US Patent Application No. 62/747,826) related to this work. T.D. and N.A. have a financial interest in GenCirq, Inc..
Figures




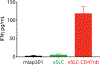







Comment in
-
Programmable bacteria as cancer therapy.Nat Med. 2019 Jul;25(7):1030-1031. doi: 10.1038/s41591-019-0513-4. Nat Med. 2019. PMID: 31270505 No abstract available.
Similar articles
-
Immunotherapy with engineered bacteria by targeting the STING pathway for anti-tumor immunity.Nat Commun. 2020 Jun 1;11(1):2739. doi: 10.1038/s41467-020-16602-0. Nat Commun. 2020. PMID: 32483165 Free PMC article.
-
Elimination of tumor by CD47/PD-L1 dual-targeting fusion protein that engages innate and adaptive immune responses.MAbs. 2018 Feb/Mar;10(2):315-324. doi: 10.1080/19420862.2017.1409319. Epub 2017 Dec 20. MAbs. 2018. PMID: 29182441 Free PMC article.
-
Engineered Bacterial Outer Membrane Vesicles as Controllable Two-Way Adaptors to Activate Macrophage Phagocytosis for Improved Tumor Immunotherapy.Adv Mater. 2022 Oct;34(40):e2206200. doi: 10.1002/adma.202206200. Epub 2022 Sep 4. Adv Mater. 2022. PMID: 35985666
-
Tumor Microenvironment-Activatable Prodrug Vesicles for Nanoenabled Cancer Chemoimmunotherapy Combining Immunogenic Cell Death Induction and CD47 Blockade.Adv Mater. 2019 Apr;31(14):e1805888. doi: 10.1002/adma.201805888. Epub 2019 Feb 14. Adv Mater. 2019. PMID: 30762908 Review.
-
Cancer immunotherapy targeting the CD47/SIRPα axis.Eur J Cancer. 2017 May;76:100-109. doi: 10.1016/j.ejca.2017.02.013. Epub 2017 Mar 10. Eur J Cancer. 2017. PMID: 28286286 Review.
Cited by
-
Effects of intratumoral microbiota on tumorigenesis, anti-tumor immunity, and microbe-based cancer therapy.Front Oncol. 2024 Sep 26;14:1429722. doi: 10.3389/fonc.2024.1429722. eCollection 2024. Front Oncol. 2024. PMID: 39391251 Free PMC article. Review.
-
Biosynthetic neoantigen displayed on bacteria derived vesicles elicit systemic antitumour immunity.J Extracell Vesicles. 2022 Dec;11(12):e12289. doi: 10.1002/jev2.12289. J Extracell Vesicles. 2022. PMID: 36468941 Free PMC article.
-
Current Status and Future Directions of Bacteria-Based Immunotherapy.Front Immunol. 2022 Jun 10;13:911783. doi: 10.3389/fimmu.2022.911783. eCollection 2022. Front Immunol. 2022. PMID: 35757741 Free PMC article. Review.
-
Probiotic-guided CAR-T cells for solid tumor targeting.Science. 2023 Oct 13;382(6667):211-218. doi: 10.1126/science.add7034. Epub 2023 Oct 12. Science. 2023. PMID: 37824640 Free PMC article.
-
Microbiome as an immune regulator in health, disease, and therapeutics.Adv Drug Deliv Rev. 2022 Sep;188:114400. doi: 10.1016/j.addr.2022.114400. Epub 2022 Jun 16. Adv Drug Deliv Rev. 2022. PMID: 35718251 Free PMC article. Review.
References
-
- Ruder WC, Lu T & Collins JJ Synthetic biology moving into the clinic. Science 333, 1248–1252 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

