O-O Bond Formation and Liberation of Dioxygen Mediated by N5 -Coordinate Non-Heme Iron(IV) Complexes
- PMID: 31271694
- PMCID: PMC6772150
- DOI: 10.1002/anie.201903902
O-O Bond Formation and Liberation of Dioxygen Mediated by N5 -Coordinate Non-Heme Iron(IV) Complexes
Abstract
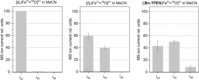
Formation of the O-O bond is considered the critical step in oxidative water cleavage to produce dioxygen. High-valent metal complexes with terminal oxo (oxido) ligands are commonly regarded as instrumental for oxygen evolution, but direct experimental evidence is lacking. Herein, we describe the formation of the O-O bond in solution, from non-heme, N5 -coordinate oxoiron(IV) species. Oxygen evolution from oxoiron(IV) is instantaneous once meta-chloroperbenzoic acid is administered in excess. Oxygen-isotope labeling reveals two sources of dioxygen, pointing to mechanistic branching between HAT (hydrogen atom transfer)-initiated free-radical pathways of the peroxides, which are typical of catalase-like reactivity, and iron-borne O-O coupling, which is unprecedented for non-heme/peroxide systems. Interpretation in terms of [FeIV (O)] and [FeV (O)] being the resting and active principles of the O-O coupling, respectively, concurs with fundamental mechanistic ideas of (electro-) chemical O-O coupling in water oxidation catalysis (WOC), indicating that central mechanistic motifs of WOC can be mimicked in a catalase/peroxidase setting.
Keywords: O−O activation; bioinorganic chemistry; iron; nitrogen ligands; oxo ligands.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Wiechen M., Nojafpour M. M., Allakhverdier S. I., Spiccia L., Energy Environ. Sci. 2014, 7, 2203–2212.
-
- Dau H., Limberg C., Reier T., Risch M., Roggan S., Strasser P., ChemCatChem 2010, 2, 724–761.
-
- Fukuzumi S., Lee Y.-M., Nam W., Dalton Trans. 2019, 48, 779–798. - PubMed
-
- Kundu S., Matito E., Walleck S., Pfaff F. F., Heims F., Rábay B., Luis J. M., Company A., Braun B., Glaser T., Ray K., Chem. Eur. J. 2012, 18, 2787–2791. - PubMed
-
- Concepcion J. J., Jurss J. W., Templeton J. L., Meyer T. J., J. Am. Chem. Soc. 2008, 130, 16462–16463. - PubMed
Publication types
Grants and funding
- GR 1247/7-1/Deutsche Forschungsgemeinschaft/International
- Berlin Cluster of Excellence UniCat/Deutsche Forschungsgemeinschaft/International
- SFB 658, Elementary Processes in Molecular Switches at Surfaces/Deutsche Forschungsgemeinschaft/International
- GR 1247/7-1/California Department of Fish and Game/International
- Berlin Cluster of Excellence UniCat/California Department of Fish and Game/International
LinkOut - more resources
Full Text Sources

