Overexpression of Nanog in amniotic fluid-derived mesenchymal stem cells accelerates dermal papilla cell activity and promotes hair follicle regeneration
- PMID: 31273189
- PMCID: PMC6802618
- DOI: 10.1038/s12276-019-0266-7
Overexpression of Nanog in amniotic fluid-derived mesenchymal stem cells accelerates dermal papilla cell activity and promotes hair follicle regeneration
Abstract
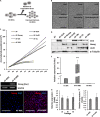
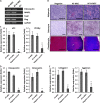
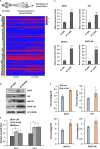
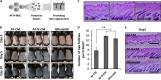
Alopecia, one of the most common chronic diseases, can seriously affect a patient's psychosocial life. Dermal papilla (DP) cells serve as essential signaling centers in the regulation of hair growth and regeneration and are associated with crosstalk between autocrine/paracrine factors and the surrounding environment. We previously demonstrated that amniotic fluid-derived mesenchymal stem cell-conditioned medium (AF-MSC-CM) accelerates hair regeneration and growth. The present study describes the effects of overexpression of a reprogramming factor, Nanog, on MSC properties, the paracrine effects on DP cells, and in vivo hair regrowth. First, we examined the in vitro proliferation and lifespan of AF-MSCs overexpressing reprogramming factors, including Oct4, Nanog, and Lin28, alone or in combination. Among these factors, Nanog was identified as a key factor in maintaining the self-renewal capability of AF-MSCs by delaying cellular senescence, increasing the endogenous expression of Oct4 and Sox2, and preserving stemness. Next, we evaluated the paracrine effects of AF-MSCs overexpressing Nanog (AF-N-MSCs) by monitoring secretory molecules related to hair regeneration and growth (IGF, PDGF, bFGF, and Wnt7a) and proliferation of DP cells. In vivo studies revealed that CM derived from AF-N-MSCs (AF-N-CM) accelerated the telogen-to-anagen transition in hair follicles (HFs) and increased HF density. The expression of DP and HF stem cell markers and genes related to hair induction were higher in AF-N-CM than in CM from AF-MSCs (AF-CM). This study suggests that the secretome from autologous MSCs overexpressing Nanog could be an excellent candidate as a powerful anagen inducer and hair growth stimulator for the treatment of alopecia.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Additive effect of bFGF and selenium on expansion and paracrine action of human amniotic fluid-derived mesenchymal stem cells.Stem Cell Res Ther. 2018 Nov 8;9(1):293. doi: 10.1186/s13287-018-1058-z. Stem Cell Res Ther. 2018. PMID: 30409167 Free PMC article.
-
Treatment of MSCs with Wnt1a-conditioned medium activates DP cells and promotes hair follicle regrowth.Sci Rep. 2014 Jun 25;4:5432. doi: 10.1038/srep05432. Sci Rep. 2014. PMID: 24961246 Free PMC article.
-
Long-term cultivation of human amniotic fluid stem cells: The impact on proliferative capacity and differentiation potential.J Cell Biochem. 2020 Jul;121(7):3491-3501. doi: 10.1002/jcb.29623. Epub 2020 Jan 3. J Cell Biochem. 2020. PMID: 31898359
-
Regenerative medicine strategies for hair growth and regeneration: A narrative review of literature.Regen Ther. 2022 Oct 31;21:527-539. doi: 10.1016/j.reth.2022.10.005. eCollection 2022 Dec. Regen Ther. 2022. PMID: 36382136 Free PMC article. Review.
-
The dermal papilla: an instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle.Cold Spring Harb Perspect Med. 2014 Jul 1;4(7):a015180. doi: 10.1101/cshperspect.a015180. Cold Spring Harb Perspect Med. 2014. PMID: 24985131 Free PMC article. Review.
Cited by
-
Mesenchymal Stem Cell Secretome for Dermatology Application: A Review.Clin Cosmet Investig Dermatol. 2021 Oct 5;14:1401-1412. doi: 10.2147/CCID.S331044. eCollection 2021. Clin Cosmet Investig Dermatol. 2021. PMID: 34675575 Free PMC article. Review.
-
Human Liver MSCs Retain Their Basic Cellular Properties in Chronically Inflamed Liver Tissue.Int J Mol Sci. 2024 Dec 13;25(24):13374. doi: 10.3390/ijms252413374. Int J Mol Sci. 2024. PMID: 39769138 Free PMC article.
-
Applications of Mesenchymal Stem Cells in Skin Regeneration and Rejuvenation.Int J Mol Sci. 2021 Feb 27;22(5):2410. doi: 10.3390/ijms22052410. Int J Mol Sci. 2021. PMID: 33673711 Free PMC article. Review.
-
TMT-Based Quantitative Proteomic Analysis Reveals the Effect of Bone Marrow Derived Mesenchymal Stem Cell on Hair Follicle Regeneration.Front Pharmacol. 2021 Jun 14;12:658040. doi: 10.3389/fphar.2021.658040. eCollection 2021. Front Pharmacol. 2021. PMID: 34194323 Free PMC article.
-
Combination of Autophagy and Stem Cell Enhancing Properties of Natural Product Extracts in Human Dermal Papilla Stem Cells.In Vivo. 2024 Jul-Aug;38(4):1767-1774. doi: 10.21873/invivo.13627. In Vivo. 2024. PMID: 38936924 Free PMC article.
References
-
- Rinaldi S, Bussa M, Mascaro A. Update on the treatment of androgenetic alopecia. Eur. Rev. Med. Pharmacol. Sci. 2016;20:54–58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

