Induction of memory-like dendritic cell responses in vivo
- PMID: 31273203
- PMCID: PMC6609631
- DOI: 10.1038/s41467-019-10486-5
Induction of memory-like dendritic cell responses in vivo
Abstract
Dendritic cells (DCs), a vital component of the innate immune system, are considered to lack antigen specificity and be devoid of immunological memory. Strategies that can induce memory-like responses from innate cells can be utilized to elicit protective immunity in immune deficient persons. Here we utilize an experimental immunization strategy to modulate DC inflammatory and memory-like responses against an opportunistic fungal pathogen that causes significant disease in immunocompromised individuals. Our results show that DCs isolated from protectively immunized mice exhibit enhanced transcriptional activation of interferon and immune signaling pathways. We also show long-term memory-like cytokine responses upon subsequent challenge with the fungal pathogen that are abrogated with inhibitors of specific histone modifications. Altogether, our study demonstrates that immunization strategies can be designed to elicit memory-like DC responses against infectious disease.
Conflict of interest statement
The authors declare no competing interests.
Figures

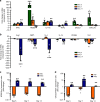

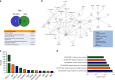


References
-
- Steinman RM. Dendritic cells and the control of immunity: enhancing the efficiency of antigen presentation. Mt. Sinai J. Med., New Y. 2001;68:160–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI071752/AI/NIAID NIH HHS/United States
- R25 GM060655/GM/NIGMS NIH HHS/United States
- 2RO1AI071752/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)/International
- GM060655/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)/International
LinkOut - more resources
Full Text Sources

