Effect of oophorosalpingo-hysterectomy on serum antioxidant enzymes in female dogs
- PMID: 31273281
- PMCID: PMC6609779
- DOI: 10.1038/s41598-019-46204-w
Effect of oophorosalpingo-hysterectomy on serum antioxidant enzymes in female dogs
Abstract
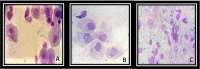
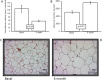
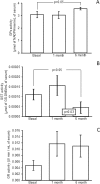
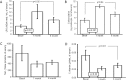
There are few studies evaluating the oxidant-antioxidant status after oophorosalpingohysterectomy (OSH) in female dogs. Here we determined the effect of OSH on antioxidant enzymes in serum, and quantified morphological changes in subcutaneous adipocytes. Lateral OSH was performed in 12 female dogs. The concentration of 17β-estradiol (17β-E2), the activities of extracellular superoxide dismutase (SOD-ec), glutathione peroxidase (GPx), glutathione-S-transferase (GST) and glutathione reductase (GR) were determined. Glutathione (GSH), glutathione disulfide (GSSG), lipid peroxidation (LPO), total antioxidant capacity (TAC), carbonylation and vitamin C were measured in serum. Subcutaneous adipose tissue was obtained to determine morphological changes and cell number, under basal conditions and six months after OSH. The SOD-ec, GPx and GST activities increased significantly (p ≤ 0.05), LPO, carbonylation and GSSG also increased. GSH and vitamin C decreased (p = 0.03). 17β-E2 tended to decrease six months after OSH. Hypertrophy of subcutaneous adipocytes was observed after OSH from the first month and was accentuated after six months (p = 0.001). The results suggest that 17β-E2 decreases after OSH and alters the antioxidant enzyme activities in serum thus, redox balance is altered. These changes are associated with an increase in body weight and hypertrophy of subcutaneous adipose tissue.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Effect of Cross-Sex Hormonal Replacement on Antioxidant Enzymes in Rat Retroperitoneal Fat Adipocytes.Oxid Med Cell Longev. 2016;2016:1527873. doi: 10.1155/2016/1527873. Epub 2016 Aug 18. Oxid Med Cell Longev. 2016. PMID: 27630756 Free PMC article.
-
Chronic cold exposure affects the antioxidant defense system in various rat tissues.Clin Chim Acta. 2003 Jul 1;333(1):69-77. doi: 10.1016/s0009-8981(03)00171-2. Clin Chim Acta. 2003. PMID: 12809737
-
Nickel-induced renal lipid peroxidation in different strains of mice: concurrence with nickel effect on antioxidant defense systems.Toxicol Lett. 1991 Oct;58(2):121-33. doi: 10.1016/0378-4274(91)90166-4. Toxicol Lett. 1991. PMID: 1949071
-
Infusion of Hibiscus sabdariffa L. Modulates Oxidative Stress in Patients with Marfan Syndrome.Mediators Inflamm. 2016;2016:8625203. doi: 10.1155/2016/8625203. Epub 2016 Jun 16. Mediators Inflamm. 2016. PMID: 27413258 Free PMC article.
-
Studies on the protective role of vitamin C and E against polychlorinated biphenyl (Aroclor 1254)--induced oxidative damage in Leydig cells.Free Radic Res. 2005 Nov;39(11):1259-72. doi: 10.1080/10715760500308154. Free Radic Res. 2005. PMID: 16298753
Cited by
-
Impact of Ovarian Suspensory Ligament Rupture on Surgical Stress in Elective Ovariohysterectomy in Bitches.Vet Sci. 2024 Dec 16;11(12):658. doi: 10.3390/vetsci11120658. Vet Sci. 2024. PMID: 39728998 Free PMC article.
-
The Protective Effect of Exogenous 17β-Estradiol against Experimentally Induced Oxidative Damage to Membrane Lipids Is Stronger in Male vs. Female Porcine Thyroids: Preliminary Results.Toxics. 2023 Sep 1;11(9):746. doi: 10.3390/toxics11090746. Toxics. 2023. PMID: 37755756 Free PMC article.
-
Nitrosative Stress and Its Association with Cardiometabolic Disorders.Molecules. 2020 May 31;25(11):2555. doi: 10.3390/molecules25112555. Molecules. 2020. PMID: 32486343 Free PMC article. Review.
-
Antioxidants and pentoxifylline as coadjuvant measures to standard therapy to improve prognosis of patients with pneumonia by COVID-19.Comput Struct Biotechnol J. 2021;19:1379-1390. doi: 10.1016/j.csbj.2021.02.009. Epub 2021 Feb 27. Comput Struct Biotechnol J. 2021. PMID: 33680348 Free PMC article.
-
Oxidant/Antioxidant Profile in the Thoracic Aneurysm of Patients with the Loeys-Dietz Syndrome.Oxid Med Cell Longev. 2020 Mar 23;2020:5392454. doi: 10.1155/2020/5392454. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32273946 Free PMC article.
References
-
- Baños. G., Guarner, V., & Pérez-Torres, I. Sex steroid hormones, cardiovascular diseases and the metabolic syndrome. Cardiovas. Hematol. Agents. Med. Chem. 9, 137–146, PMID: 21745183 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

